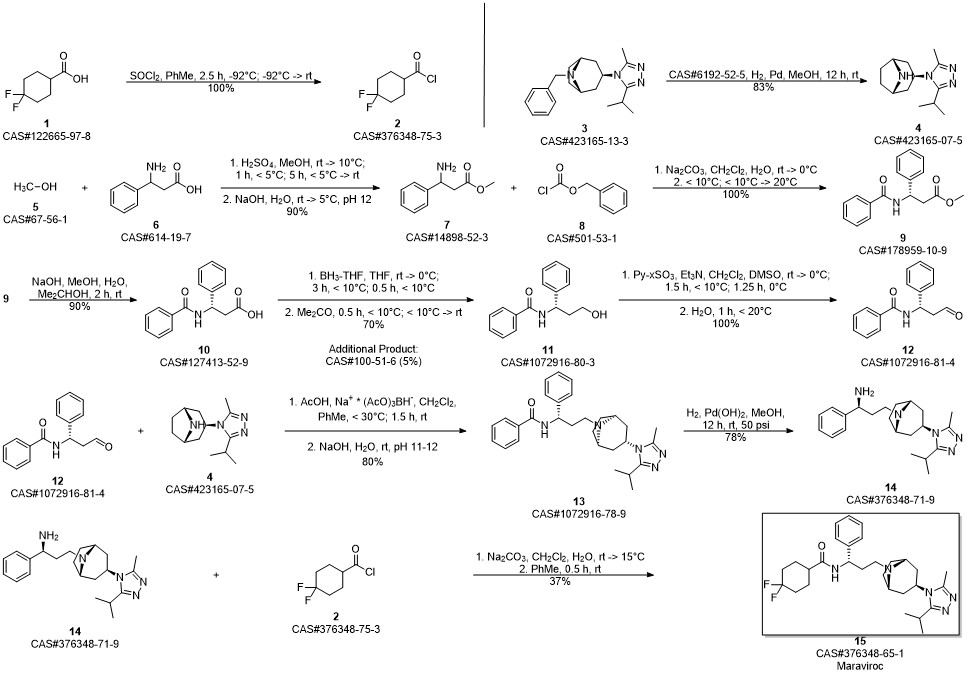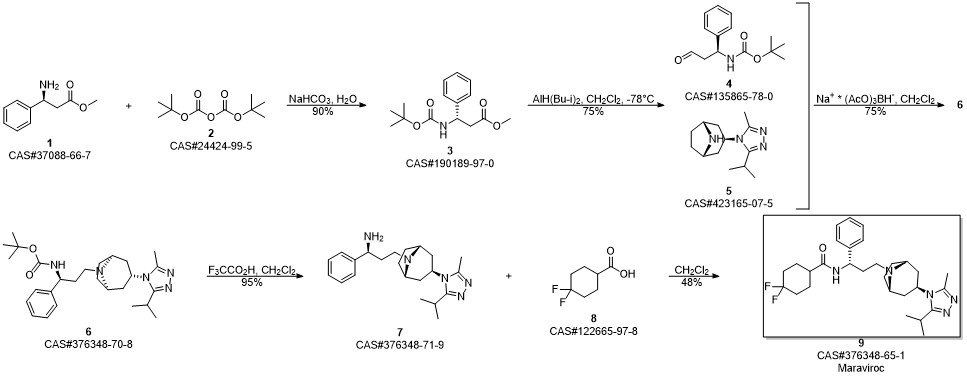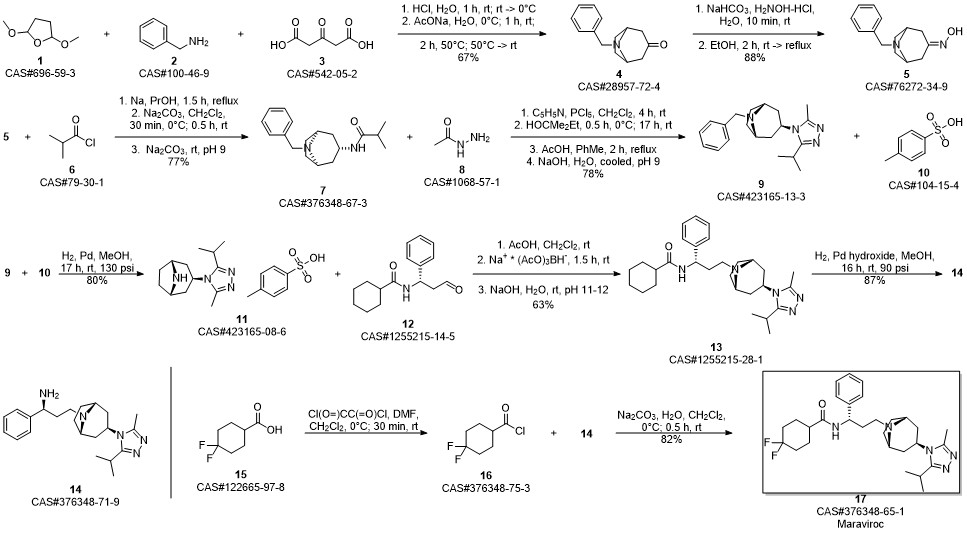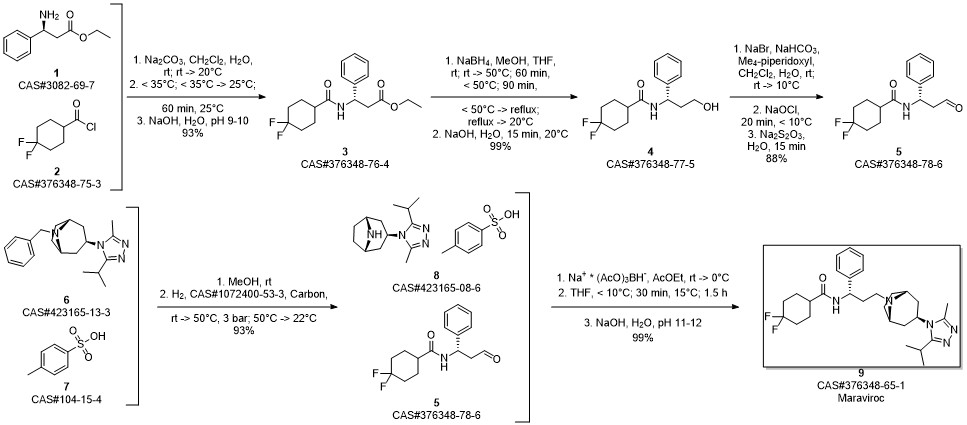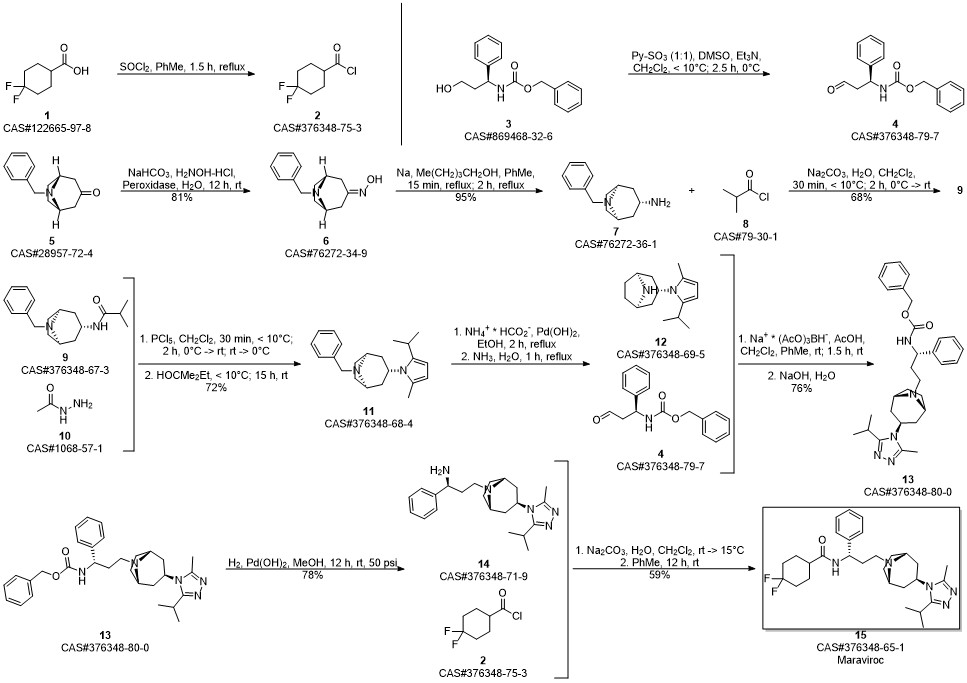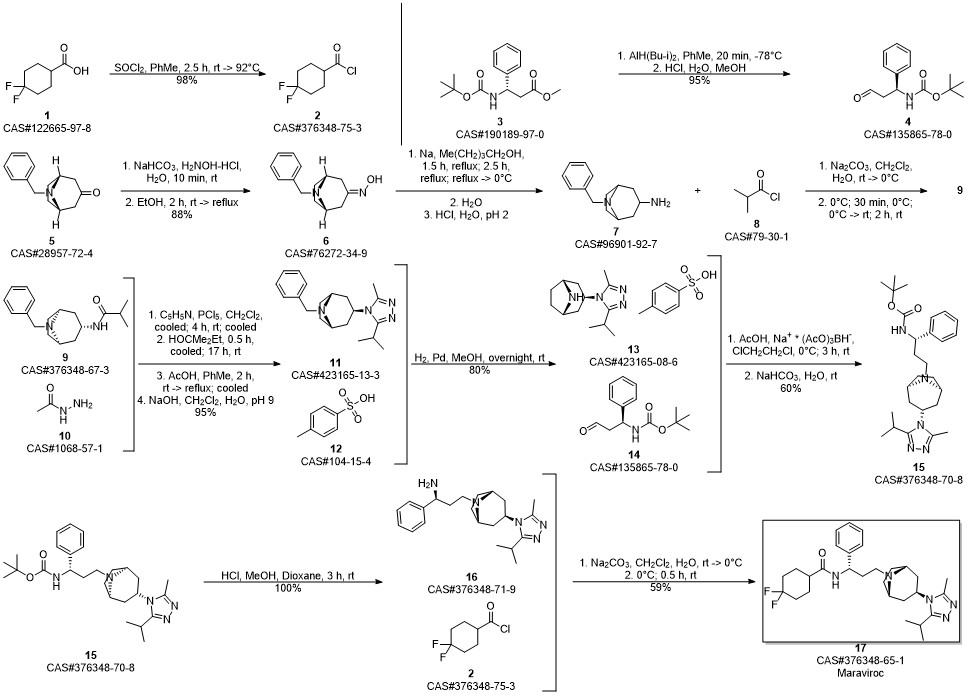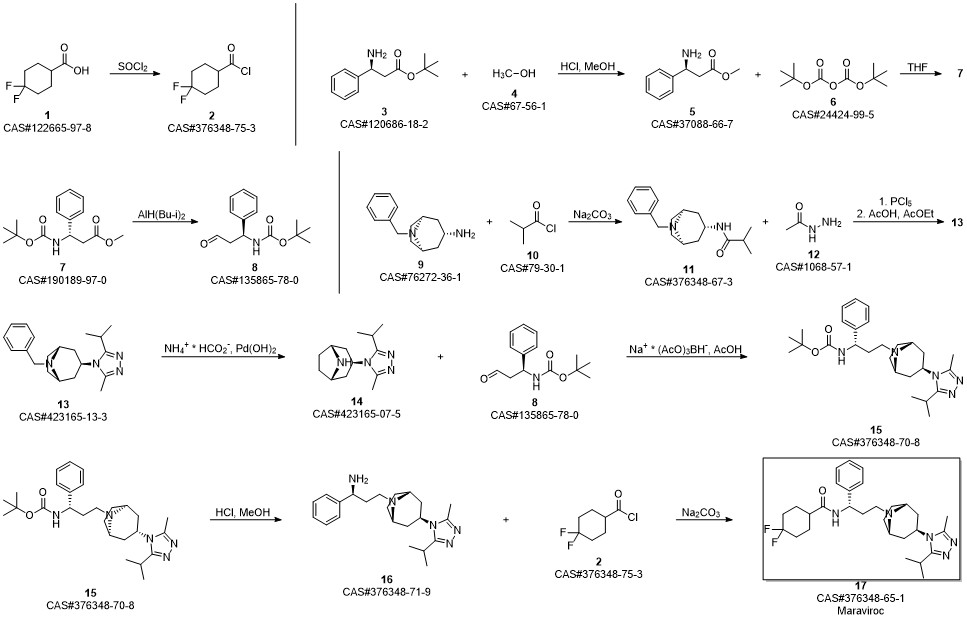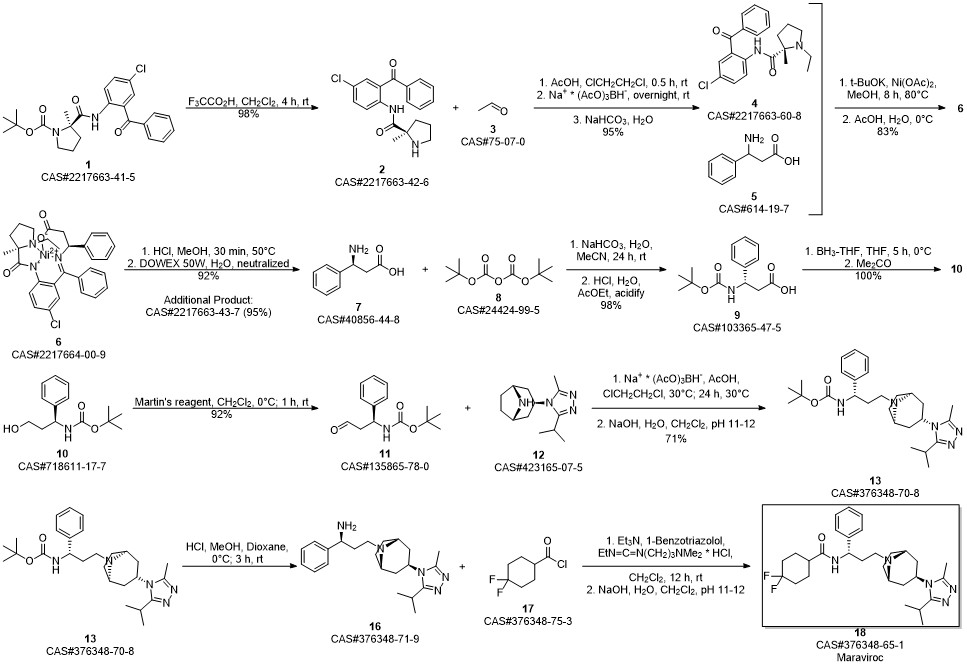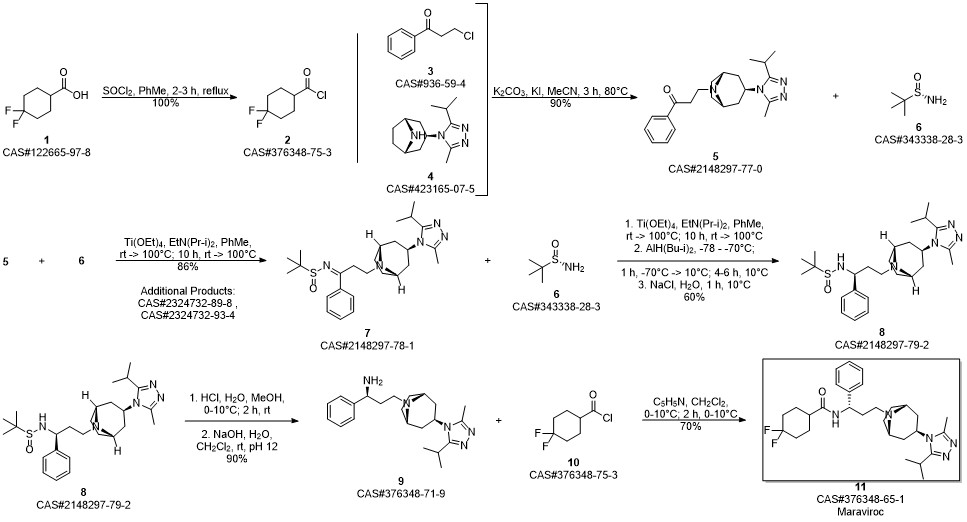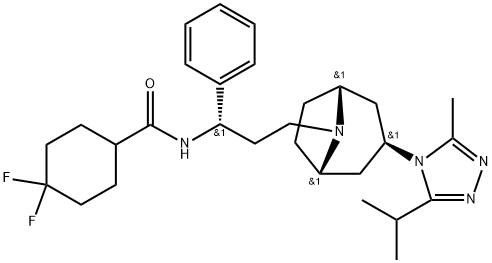
Maraviroc synthesis
- Product Name:Maraviroc
- CAS Number:376348-65-1
- Molecular formula:C29H41F2N5O
- Molecular Weight:513.68
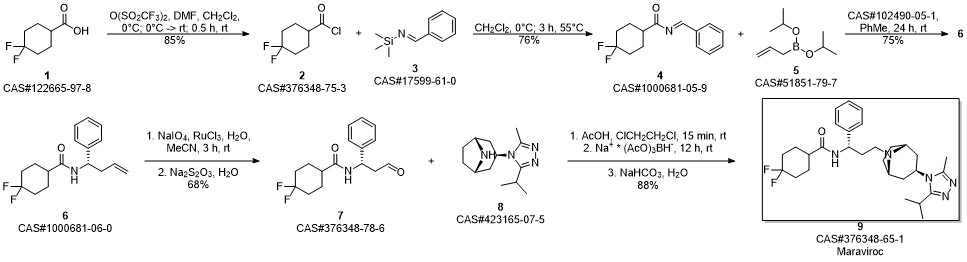
Lou, Sha; Moquist, Philip N.; Schaus, Scott E. Asymmetric Allylboration of Acyl Imines Catalyzed by Chiral Diols. Journal of the American Chemical Society. Volume 129. Issue 49. Pages 15398-15404. 2007.
![(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/CAS/GIF/423165-07-5.gif)
423165-07-5
114 suppliers
$60.00/100mg
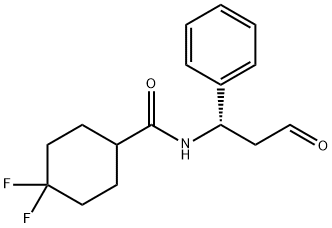
376348-78-6
31 suppliers
inquiry

376348-65-1
240 suppliers
$35.00/5mg
Yield:376348-65-1 4.6 g
Reaction Conditions:
Stage #1:UK-408027;4,4-difluorocyclohexanecarboxylic acid (3-oxo-1-phenyl-propyl)-amide in tetrahydrofuran for 0.166667 h;Cooling with ice;
Stage #2: with acetic acid in tetrahydrofuran for 0.166667 h;
Stage #3: with sodium tris(acetoxy)borohydride in tetrahydrofuran
Steps:
6 Example 6: Preparation of Maraviroc
Compound (I) (3.09 g) as prepared in Example 5, compound (II) (2.34 g) as prepared in Example 4, and THF (30 g) were mixed in a round bottom flask. After the mixture was cooled in an ice bath and reacted for 10 min, HOAc (1.5 g) was added into the mixture and reacted for another 10 min. Subsequently, NaBH(OAc)2 (3.2 g) was added into the reaction mixture to reduce intermediate to crude product. The whole reaction mixture was quenched with 15% NaOH to pH >10. The organic phase was collected by phase separation, and concentrated under reduced pressure. Maraviroc (4.6 g) in a form of white powder was obtained from the concentrate by crystallization with EA.
References:
SCI Pharmtech, Inc.;Wang, Heng-Yen;Kao, Chen-Yi US2019/248782, 2019, A1 Location in patent:Paragraph 0030
![(1S)-3-[3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-exo-8-azabicyclo[3.2.1]oct-8-yl]-1-phenyl-1-propanamine](/CAS2/GIF/376348-71-9.gif)
376348-71-9
20 suppliers
$135.00/5MG
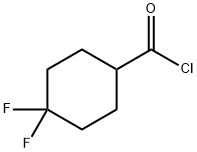
376348-75-3
28 suppliers
$90.00/50mg

376348-65-1
240 suppliers
$35.00/5mg
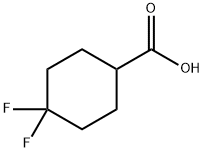
122665-97-8
285 suppliers
$18.00/250mg
![(1S)-3-[3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-exo-8-azabicyclo[3.2.1]oct-8-yl]-1-phenyl-1-propanamine](/CAS2/GIF/376348-71-9.gif)
376348-71-9
20 suppliers
$135.00/5MG

376348-65-1
240 suppliers
$35.00/5mg

376348-78-6
31 suppliers
inquiry
![3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane-p-toluenesulfonate](/CAS2/GIF/423165-08-6.gif)
423165-08-6
32 suppliers
$188.00/250mg

376348-65-1
240 suppliers
$35.00/5mg

122665-97-8
285 suppliers
$18.00/250mg

376348-65-1
240 suppliers
$35.00/5mg