Imidacloprid synthesis
- Product Name:Imidacloprid
- CAS Number:138261-41-3
- Molecular formula:C9H10ClN5O2
- Molecular Weight:255.66
Technical production starts with the Tschitschibabin reaction of 3-methylpyridine giving 2-amino-5-methylpyridine (14), which is transformed to the chloride (15) by the Sandmeyer reaction in the presence of hydrogen chloride. A successive operation of chlorination of the methyl group to 10 and the subsequent substitution of the active chloride with ethylene diamine to 16 are carried out without isolation of the intermediates. The final product is produced by ring formation with nitroguanidine. This multistep process affords the product at a purity of >95% (41).
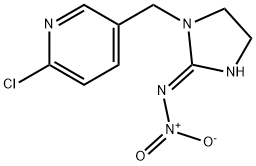
101990-44-7
5 suppliers
inquiry

138261-41-3
477 suppliers
$29.00/25mg
Yield:138261-41-3 80%
Reaction Conditions:
in dichloromethane;
Steps:
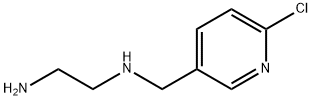
5 1-(2-Chloro-5-pyridylmethyl)-2-(nitroimino)imidazolidine:
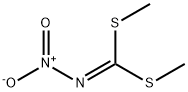
(Example 5.) 1-(2-Chloro-5-pyridylmethyl)-2-(nitroimino)imidazolidine: To a solution of 2.50 g (15 mmol) of dimethyl N-ni troimidodithiocarbonate in 10 ml of dichloromethane, 2.78 a (15 mmol) of N-(2-chloro-5-pyridylmethyl)ethylenediamine was added with stirring at 25 to 32°C under ice cooling. The reaction mixture was stirred at room temperature for more 3 hours, followed by evaporating the solvent under reduced pressure. Crude crystal deposited was recrystallized from ethanol and dried. Thus, 3.07 g of the desired compound was obtained, The yield was 80.0 %. Melting point 134-6 °C.
References:
EP500952,1992,A1

101990-44-7
5 suppliers
inquiry
141972-53-4
0 suppliers
inquiry

138261-41-3
477 suppliers
$29.00/25mg
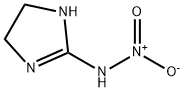
5465-96-3
224 suppliers
$26.57/5G
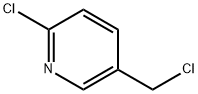
70258-18-3
515 suppliers
$5.00/5g

138261-41-3
477 suppliers
$29.00/25mg
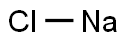
7647-14-5
1146 suppliers
$5.00/500g

5465-96-3
224 suppliers
$26.57/5G

70258-18-3
515 suppliers
$5.00/5g

138261-41-3
477 suppliers
$29.00/25mg