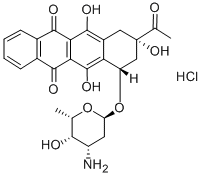
Idarubicin hydrochloride synthesis
- Product Name:Idarubicin hydrochloride
- CAS Number:57852-57-0
- Molecular formula:C26H28ClNO9
- Molecular Weight:533.95
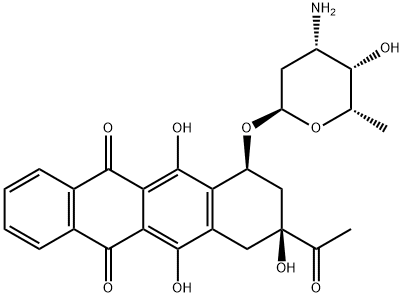
58957-92-9
117 suppliers
inquiry

57852-57-0
243 suppliers
$31.00/1mg
Yield:57852-57-0 95%
Reaction Conditions:
with hydrogenchloride in chloroform;isopropyl alcohol;
Steps:
2
Example 210091] 1 g idarubicin free base was introduced into 100 ml of a mixture of 80 ml chloroform and 20 ml methanol. The pH value of this mixture was then set to a value in the range of 3.5-4.0 by adding 0.1 M isopropanolic HC1 solution. This mixture was mixed with 100 ml 1 -butanol 10 ml watet Then, the chloroform was slowly removed from the mixture by distillation at 60° C. Thereafter, 20 ml of this mixture was slowly removed by distillation in a vacuum at 80° C., in order to reduce the water content to less than 4.0 volume percent, relative to the total volume of the mixture. Here, a suspension formed that was cooled to 20° C. within 6 hours. At this temperature, the suspension was stirred for an additional 12 hours. The crystals contained in the suspension as solids were filtered and washed with 20 ml acetone. The crystals were then dried for 12 hours under vacuum. A yield of idarubicinhydrochloride of 95% resulted.
References:
US2014/228311,2014,A1 Location in patent:Paragraph 0091
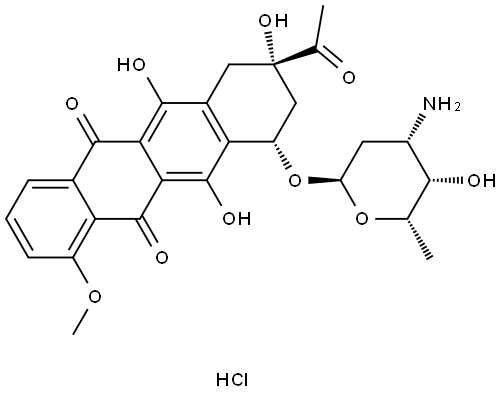
![5,12-Naphthacenedione, 9-acetyl-7,8,9,10-tetrahydro-6,9,11-trihydroxy-7-[[2,3,6-trideoxy-3-[(2,2,2-trifluoroacetyl)amino]-α-L-lyxo-hexopyranosyl]oxy]-, (7S,9S)-](/CAS/20211123/GIF/60660-74-4.gif)
60660-74-4
1 suppliers
inquiry

57852-57-0
243 suppliers
$31.00/1mg
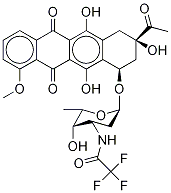
23541-50-6
475 suppliers
$15.00/10mg

57852-57-0
243 suppliers
$31.00/1mg
26388-52-3
14 suppliers
$90.00/1mg

57852-57-0
243 suppliers
$31.00/1mg
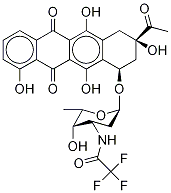
68594-06-9
14 suppliers
$124.90/1mg

57852-57-0
243 suppliers
$31.00/1mg