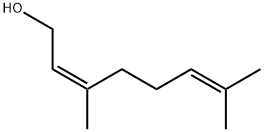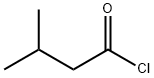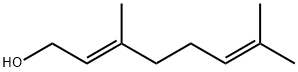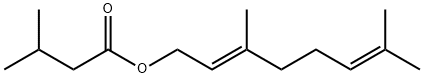
GERANYL ISOVALERATE synthesis
- Product Name:GERANYL ISOVALERATE
- CAS Number:109-20-6
- Molecular formula:C15H26O2
- Molecular Weight:238.37
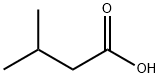
503-74-2
418 suppliers
$5.00/10g
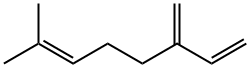
123-35-3
414 suppliers
$25.19/5ml
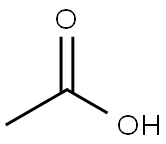
64-19-7
1560 suppliers
$10.00/25ML
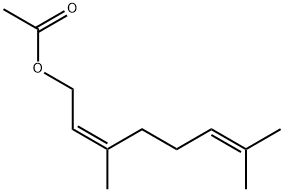
141-12-8
271 suppliers
$7.00/5g
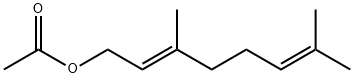
105-87-3
418 suppliers
$8.00/25g
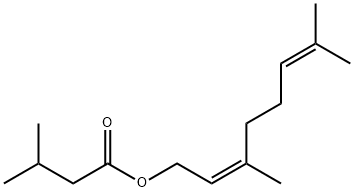
3915-83-1
14 suppliers
inquiry

109-20-6
88 suppliers
$28.00/5G
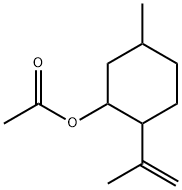
89-49-6
20 suppliers
inquiry
138-86-3
332 suppliers
$22.00/25mL
Yield:-
Reaction Conditions:
with sodium acetate in methoxybenzene under 760.051 Torr; for 18 h;Product distribution / selectivity;Heating / reflux;
Steps:
V
Example V; Preparation of Geranyl/Neryl Esters by Treating Myrcene with a mixture of Alkanoic Acids; Using a procedure similar to that described in Example I, a mixture of 0.25 ml (200 mg, 1.47 mmoles) of myrcene, 1.00 ml of me, thoxybenzene ("anisole." purchased from Fisher Scientific Co. ), 1.00 ml (9.2 mmoles) of isovaleric acid (purchased from Aldrich Chemcial Co., Milwaukee, Wisconsin), 3.00 ml (52. 4 mmoles) of glacial acetic acid, and 96 mg (1.17 mmoles) of anhydrous sodium acetate was heated at vigorous reflux (atmospheric pressure) for 18 hours. After cooling the mixture at room temperature, the product was isolated as described in the procedure of Example I. Most of the anisole (along with some of the"unreactd"myrcene) was removed at reduced-pressure (as low as 1 mmHg) over a period of approximately 40 minutes. The residual material (196 mg), which contained traces of anisole along with unreacted myrcene, was subjected to analysis by recording a proton NMR spectrum of this mixture (CDC13 solution, 400 bEz) and integrating the areas of signals arising from each of the components known to be in the mixture. This analysis indicated that the reaction proceeded more slowly in the presence of both a co-solvent and the less polar (vs. acetic acid) C-5 alkanoic acid, i. e. , approximately 8% conversion of myrcene to a 6 : 1 mixture of geranyl/neryl acetate : geranyl/heryl isovalerate. The latter ester was characterized by a doublet (J = 6.4 Hz) at 8 0. 94 tHC (CH3) 2, 6H's] and a doublet (J = 7. 6 Hz) at 6 4.59 (CH2O). Although the process was slow, by-products such as alpha-terpinyl esters and limonene ("dipentene") were formed to a lesser extent in the less polar reaction mixture. Although the above experimental conditions result in the formation of two geranyl esters (geranyl acetate and geranyl isovalerate), if one intends to manufacture geraniol, and subsequently oxidize it to citral, such a mixture does not have to be separated. Both esters can be simultaneously hydrolyzed by water in the presence of a lipase. enzyme to yield geraniol and a mixture of water-soluble alkanoic acids
References:
LOYOLA UNIVERSITY OF CHICAGO WO2005/44774, 2005, A1 Location in patent:Page/Page column 16-17