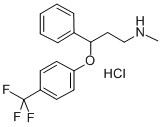
Fluoxetine hydrochloride synthesis
- Product Name:Fluoxetine hydrochloride
- CAS Number:56296-78-7
- Molecular formula:C17H19ClF3NO
- Molecular Weight:345.79
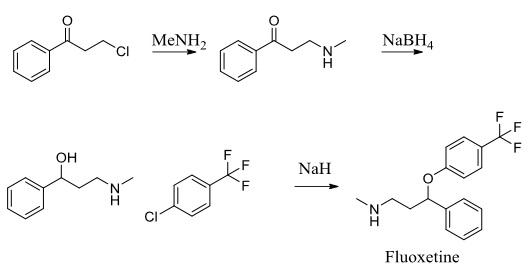
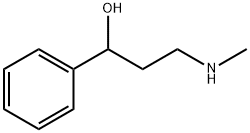
Fluoxetine is the active ingredient in the antidepressant Prozac. It works as a selective serotonin reuptake inhibitor to treat conditions including depression and obsessive-compulsive disorder. To assemble this molecule, a three-step synthesis was utilized. Intermediates included 1- propanone, 3-(methylamino)-1-phenyl-(synthesized through an SN2 reaction between 3-chloropropiophenone and methylamine) and α-[2- (methylamino) ethyl] benzyl alcohol (synthesized through reduction of the first intermediate using NaBH4). The second intermediate was subjected to 4-chlorobenzotrifluoride and sodium hydride to produce the desired molecule, fluoxetine.
42142-52-9
370 suppliers
$11.00/25g
455-13-0
246 suppliers
$10.00/1g

56296-78-7
488 suppliers
$5.00/1g
Yield:-
Reaction Conditions:
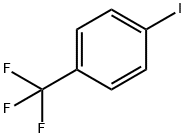
Stage #1: 3-methylamino-1-phenylpropan-1-ol;4-Iodobenzotrifluoridewith caesium carbonate;copper(I) bromide in xylenes at 130; for 16 h;
Stage #2: with hydrogenchloride in tert-butyl methyl ether;isopropyl alcohol;xylenes at 20;
Steps:
4 EXAMPLE 4
A mixture of N-methyl-3-hydroxy-3-phenylpropylamine (1.65 g, 10 mmol), 4-iodobenzotrifluoride (3.25 g, 12 mmol), cuprous bromide (0.17 g), cesium carbonate (3.9 g, 12 mmol) and xylenes (1 mL) was stirred under nitrogen at 130° C. until reaction completion as determined by 1H NMR (16 hours). The reaction mixture was cooled to room temperature, diluted with methyl t-butyl ether (20 mL), filtered, and rinsed with more methyl t-butyl ether. 20% HCl solution in isopropanol (3 mL) was added and the resulting solution was evaporated to dryness to yield a solid residue. The residue was stirred with methyl t-butyl ether (20 mL) for 1 hour at room temperature and the suspension was filtered and washed with more methyl t-butyl ether to give 2.4 g of N-methyl-3-(4-trifluoromethylphenoxy)-3-phenylpropylamine hydrochloride (Fluoxetine hydrochloride) as a white solid. 1H NMR spectrum of the product is identical to that of Example 3.
References:
US2007/10678,2007,A1 Location in patent:Page/Page column 7

42142-52-9
370 suppliers
$11.00/25g
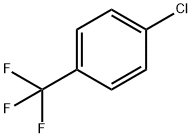
98-56-6
396 suppliers
$11.00/25g

56296-78-7
488 suppliers
$5.00/1g
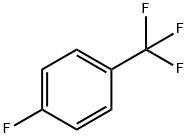
402-44-8
288 suppliers
$9.00/1g
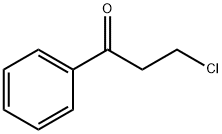
936-59-4
437 suppliers
$8.00/5g
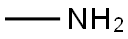
74-89-5
7 suppliers
$13.44/25ML

56296-78-7
488 suppliers
$5.00/1g
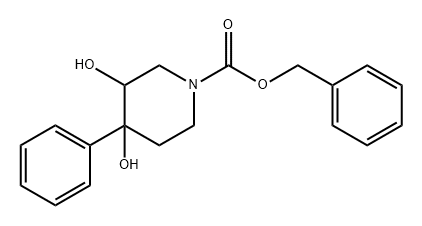
871926-94-2
0 suppliers
inquiry

56296-78-7
488 suppliers
$5.00/1g
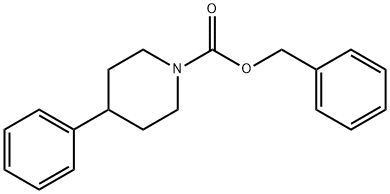
733810-73-6
22 suppliers
inquiry

56296-78-7
488 suppliers
$5.00/1g