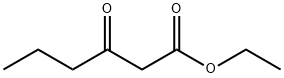
Ethyl butyrylacetate synthesis
- Product Name:Ethyl butyrylacetate
- CAS Number:3249-68-1
- Molecular formula:C8H14O3
- Molecular Weight:158.2

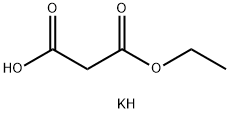
2033-24-1
661 suppliers
$6.00/25g
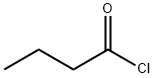
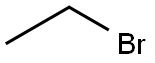
141-75-3
352 suppliers
$12.32/25G

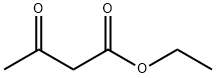
3249-68-1
249 suppliers
$6.00/5g
Yield:-
Reaction Conditions:
Stage #1: cycl-isopropylidene malonatewith pyridine in dichloromethane at 0;
Stage #2: butyryl chloride in dichloromethane at 0 - 25;
Stage #3: with hydrogenchloride in water;
Steps:
I
Synthesis of ethyl-2-bronio-4-propyl-l,3-thiazoI-5-carboxylateStep I: Synthesis of ethyl 3-oxohexanoateA ~0 0C solution of meldrum's acid (5 g., 34.7 mmol), anhydrous dichloromethane (40ml) was treated dropwise with pyridine (5.55 g, 69.4 mmol) and stirred for 15 min. Butryl chloride (4.06 g, 38 mmol, 1.1 equiv) was added dropwise while maintaining the temperature at about 0 0C, and the reaction mixture was stirred at room temperature (~25 0C) for about lhour. The reaction mixture was concentrated, added 6M HCl (100ml) and extracted with ethyl acetate. The combined organics were dried over anhydrous sodium sulphate to afford acylated meldrum's acid (7g). This was then refluxed in ethanol (50ml) for about 20 hours. The reaction mixture was concentrated to dryness, taken up in ethyl acetate (200 ml) and washed with aq. sodium bicarbonate water, brine, dried (ann. Na2SO4) and concentrated to afford the crude compound that was purified by column chromatography over SiO2 (100- 200).and eluted in 2 % EtOAc/Hexane (2 g).EIMS m/z 159 [M+H]+
References:
WO2010/13222,2010,A1 Location in patent:Page/Page column 65

2033-24-1
661 suppliers
$6.00/25g

64-17-5
748 suppliers
$10.00/50g

141-75-3
352 suppliers
$12.32/25G

3249-68-1
249 suppliers
$6.00/5g
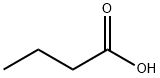
107-87-9
350 suppliers
$14.00/25mL
105-58-8
467 suppliers
$15.00/25g

3249-68-1
249 suppliers
$6.00/5g