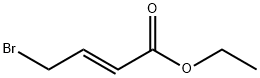
Ethyl 4-bromocrotonate synthesis
- Product Name:Ethyl 4-bromocrotonate
- CAS Number:37746-78-4
- Molecular formula:C6H9BrO2
- Molecular Weight:193.04
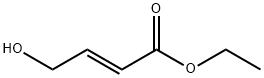
10080-68-9
17 suppliers
$504.31/5MG

37746-78-4
188 suppliers
$10.00/5g
Yield:37746-78-4 81.2%
Reaction Conditions:
with bromine;triphenylphosphine in dichloromethane at 0; for 1.5 h;
Steps:
1.2
Step 2Triphenyl phosphine (295 g, 1.12 mol) and methylene chloride (665 mL) were introduced into a 3 L one-neck flask and were stirred for 10 minutes at 0° C. And then, bromine (179.3 g) dissolved in methylene chloride (665 mL) was slowly added as droplets therein for 1 hours. At this time, the temperature of the reactor was maintained to be 0° C. The reaction product was further stirred for 30 minutes after all of bromine was added. (E)-ethyl 4-hydroxybut-2-enoate (133 g, 1.02 mol) dissolved in methylene chloride (665 mL) was slowly added as droplets into the reaction mixture for 1 hours. At this time, the temperature of the reactor was also maintained to be 0° C. After all of (E)-ethyl 4-hydroxybut-2-enoate was added, the mixture was further stirred for 30 minutes at 0° C. (the reaction was confirmed with TLC, EtOAc:hexane=1:4). After washing the organic layer of the reaction product with water (900 mL×2), the organic layer was dried with anhydrous MgSO4, and then a large quantity of Ph3PO was extracted in company with the product by filtering and concentrating the same. The triphenyl phosphine oxide was eliminated by filtering the product, after adding n-hexane (1 L) therein and stirring the same for 30 minutes with a mechanical stirrer. (E)-ethyl 4-bromobut-2-enoate of 160 g was obtained with a yield of 81.2% by concentrating the filtered solution under a decompressed condition and distillating the concentrated solution under vacuum condition.1H-NMR (δ ppm, CDCl3, 400 Mz): 6.92 (m, 1H), 5.95 (m, 1H), 4.15 (q, 2H), 3.95 (m, 2H), 1.25 (t, 3H)
References:
KOLON LIFE SCIENCE, INC. US2012/46494, 2012, A1 Location in patent:Page/Page column 5
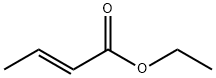
623-70-1
167 suppliers
$10.00/5g

37746-78-4
188 suppliers
$10.00/5g
91890-87-8
4 suppliers
inquiry

37746-78-4
188 suppliers
$10.00/5g
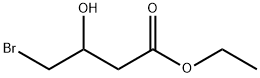
32224-01-4
7 suppliers
inquiry

37746-78-4
188 suppliers
$10.00/5g
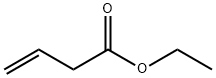
1617-18-1
60 suppliers
$98.00/100mg

37746-78-4
188 suppliers
$10.00/5g