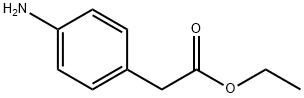
Ethyl 4-aminophenylacetate synthesis
- Product Name:Ethyl 4-aminophenylacetate
- CAS Number:5438-70-0
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
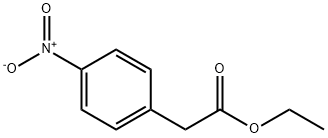
5445-26-1
172 suppliers
$10.00/1g

5438-70-0
166 suppliers
$13.00/5g
Yield:5438-70-0 100%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol for 4 h;
Steps:
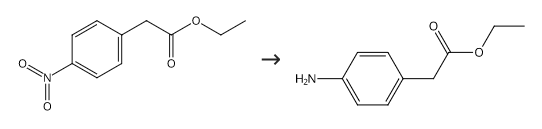
12 Ethyl 2-(4-aminophenyl)acetate (4j).
A solution of ethyl 2-(4-nitrophenyl)acetate (3j) (1.05 g, 3.40 mmol) inMeOH (25 mL) was hydrogenated over 10% Pd/C (catalytic amount) for 4h. The catalyst was filtered off and the resultingsolution was concentrated to afford ethyl 2-(4-aminophenyl)acetate (4j). 1H-NMR 200 MHz (CDCl3) δ: 1.29 (t, 3H, J=7.2Hz ), 3.72 (s, 2H), 4.13 (q, 2H, J=7.2 Hz), 6.51 (d, 2H, J= 7.2 Hz), 6.98 (d, 2H, J= 7.2 Hz). Quantitative yield
References:
Leadiant Biosciences SA;GIANNINI, Giuseppe;SIMONI, Daniele;OLIVA, Paola;MOR, Marco;RIVARA, Silvia EP3381898, 2018, A1 Location in patent:Paragraph 0200; 0272

64-17-5
724 suppliers
$10.00/50g
1197-55-3
402 suppliers
$13.00/25g

5438-70-0
166 suppliers
$13.00/5g

1197-55-3
402 suppliers
$13.00/25g

5438-70-0
166 suppliers
$13.00/5g
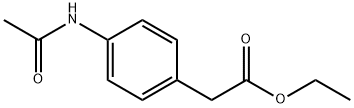
13475-17-7
57 suppliers
$27.00/5g

5438-70-0
166 suppliers
$13.00/5g
![Benzeneacetyl chloride, 4-[(2,2,2-trifluoroacetyl)amino]-](/CAS/20210111/GIF/113180-63-5.gif)
113180-63-5
0 suppliers
inquiry

5438-70-0
166 suppliers
$13.00/5g