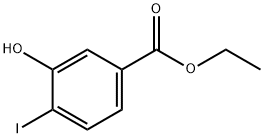
ETHYL 3-HYDROXY-4-IODOBENZOATE synthesis
- Product Name:ETHYL 3-HYDROXY-4-IODOBENZOATE
- CAS Number:203187-56-8
- Molecular formula:C9H9IO3
- Molecular Weight:292.07

64-17-5
724 suppliers
$10.00/50g
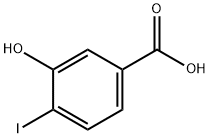
58123-77-6
190 suppliers
$6.00/250mg

203187-56-8
21 suppliers
inquiry
Yield:203187-56-8 97%
Reaction Conditions:
with hydrogenchlorideProduct distribution / selectivity;Heating / reflux;
Steps:
3
Dissolve 3-hydroxy-4-iodobenzoic acid (2.02 g, 7.65 mmol) in ethanol saturated with HCl gas (50 ml). Heat the solution under reflux overnight. Evaporate ethanol to yield 3-Hydroxy-4-iodo-benzoic acid ethyl ester compound (97% yield). Reflux 3-hydroxy-4-iodo-benzoic acid (38 g, 144 mmol) and saturated solution of HCl in ethanol (600 mL) overnight. Evaporate the solvent yielding 42 g, 99% of 3-hydroxy-4-iodo-benzoic acid ethyl ester. 1H NMR (300 MHz, CDCl3): δ 1.39 (t, J=7.1 Hz, 3H), 4.36 (q, J=7.1 Hz, 2H), 7.33 (dd, J=8.3 and 2.0 Hz, 1H), 7.64 (d, J=2.0 Hz, 1H), 7.75 (d, J=8.3 Hz, 1H). Add ethyl iodide (33.8 g, 216 mmol) to a solution of 3-hydroxy-4-iodo-benzoic acid ethyl ester (42 g, 144 mmol) and K2CO3 (39.9 g, 288 mmol) in acetonitrile (400 mL) under magnetic stirring. Heat the reaction mixture at 65° C. for 2 hours, allow the mixture to cool and maintain at RT overnight. Evaporate the solvent and add ethyl acetate to the crude. Filter the solid through Celite and evaporate the solvent. Yield 42 g, 96%. 1H NMR (300 MHz, CDCl3): δ 1.39 (t, J=7.1 Hz, 3H), 1.50 (t, J=7.0 Hz, 3H), 4.16 (q, J=7.0 Hz, 2H), 4.37 (q, J=7.1 Hz, 2H), 7.36 (dd, J=8.1 and 1.8 Hz, 1H), 7.42 (d, J=1.8 Hz, 1H), 7.84 (d, J=8.1 Hz, 1H).
References:
US2006/276532,2006,A1 Location in patent:Page/Page column 5
7781-98-8
203 suppliers
$10.00/5g

203187-56-8
21 suppliers
inquiry

58123-77-6
190 suppliers
$6.00/250mg

203187-56-8
21 suppliers
inquiry