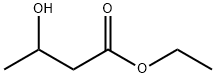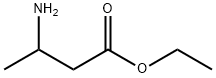
ETHYL 3-AMINOBUTYRATE synthesis
- Product Name:ETHYL 3-AMINOBUTYRATE
- CAS Number:5303-65-1
- Molecular formula:C6H13NO2
- Molecular Weight:131.17

64-17-5
724 suppliers
$10.00/50g
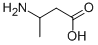
2835-82-7
131 suppliers
$638.00/0.5G

5303-65-1
89 suppliers
$49.00/5g
Yield:5303-65-1 512 mg
Reaction Conditions:
Stage #1: ethanolwith acetyl chloride at 0 - 20; for 1 h;
Stage #2: 3-aminobutyric acid for 3 h;Reflux;
Steps:
Synthesis of N-ethoxycarbonylmethyl-3-aminobutyric acid ethyl ester
Acetyl chloride (1 ml) was slowly added to ethanol (10 ml) at 0 °C. Then the solution was stirred for 1 h at room temperature. 3-Aminobutyric acid (313 mg) was added to the resulting 10% HCl in ethanol solution. After reflux for 3 h, the solvent was evaporated to provide 3-aminobutyric acid ethyl ester (512 mg). 3-Aminobutyric acid ethyl ester (134 mg) was dissolved in acetonitrile (2.5 ml). K2CO3 (356 mg) was then added to the solution. After stirring the mixture for 15 min at 0 °C, ethyl bromoacetate (121 mg) was added, and the mixture was then stirred for 17 h at room temperature. After evaporation of the solvent, the residue was dissolved in saturated NaHCO3 and extracted with ethyl acetate. After concentration in vacuo, the oil residue was purified with silica gel chromatography (hexane:ethyl acetate = 1:1) to yield N-ethoxycarbonylmethyl-3-aminobutyric acid ethyl ester (69.6 mg).
References:
Chisuga, Taichi;Miyanaga, Akimasa;Kudo, Fumitaka;Eguchi, Tadashi [Journal of Biological Chemistry,2017,vol. 292,# 26,p. 10926 - 10937]
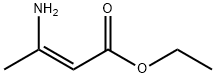
626-34-6
209 suppliers
$6.00/5g

5303-65-1
89 suppliers
$49.00/5g
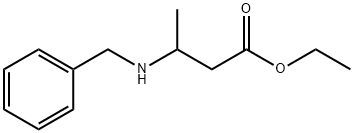
6335-80-4
10 suppliers
$368.00/1g

5303-65-1
89 suppliers
$49.00/5g
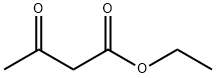
141-97-9
790 suppliers
$5.00/25g

5303-65-1
89 suppliers
$49.00/5g