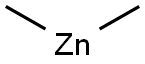
DIMETHYLZINC synthesis
- Product Name:DIMETHYLZINC
- CAS Number:544-97-8
- Molecular formula:C2H6Zn
- Molecular Weight:95.46
75-24-1
157 suppliers
$54.50/100ml
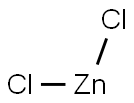
7646-85-7
561 suppliers
$10.00/25ml
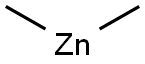
544-97-8
132 suppliers
$130.00/5g
Yield:544-97-8 92%
Reaction Conditions:
at 60; for 6 h;Inert atmosphere;
Steps:
2; 4 Example 4
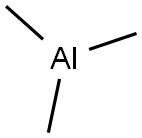
A method for preparing dimethyl zinc, comprising the steps of: adding zinc chloride to a reaction kettle under nitrogen protection, and then adding the moles of trimethylaluminum, trimethylaluminum and zinc chloride dropwise under stirring conditions. The ratio is 1: 1.8. The reaction speed is controlled by the dropping acceleration of trimethylaluminum. After the completion of the dropping of trimethylaluminum, the stirring reaction is continued for 4.5 hours, and then the temperature of the kettle is adjusted to 42 ° C and maintained for 2h. The distilled Zn is collected (CH3) 2; Then, diethyl zinc was added dropwise to the reaction kettle, and the molar ratio of trimethylaluminum and diethyl zinc was 1.3: 1, and the temperature of the reaction kettle was controlled to 91 ° C. The distilled Zn ( CH3) 2. After the addition of diethylzinc was completed, the temperature of the kettle was maintained at 91 ° C, and Zn (CH3) 2 was collected for 3 hours.The yield of dimethylzinc in this example is 92% (calculated as the methyl group of trimethylaluminum).
References:
Henan Chengming Optoelectric New Materials Co., Ltd.;Ning Hongfeng CN104774214, 2016, B Location in patent:Paragraph 0014-0016; 0020-0022

74-88-4
346 suppliers
$15.00/10g
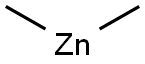
544-97-8
132 suppliers
$130.00/5g
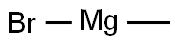
75-16-1
269 suppliers
$12.00/10ml
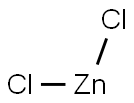
7646-85-7
561 suppliers
$10.00/25ml
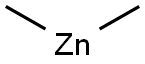
544-97-8
132 suppliers
$130.00/5g
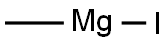
917-64-6
149 suppliers
$45.00/10ml
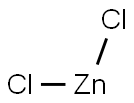
7646-85-7
561 suppliers
$10.00/25ml
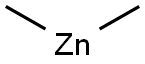
544-97-8
132 suppliers
$130.00/5g
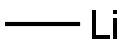
917-54-4
174 suppliers
$44.39/50ml
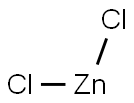
7646-85-7
561 suppliers
$10.00/25ml
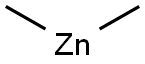
544-97-8
132 suppliers
$130.00/5g