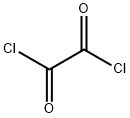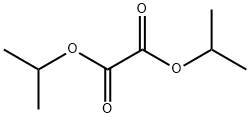
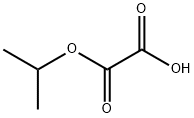
diisopropyl oxalate synthesis
- Product Name:diisopropyl oxalate
- CAS Number:615-81-6
- Molecular formula:C8H14O4
- Molecular Weight:174.19
Yield: 90%
Reaction Conditions:
with toluene-4-sulfonic acid in toluene for 24 h;Reflux;
Steps:
1 Example 1. Synthesis of Diisopropyl Oxalate from Oxalic Acid
Oxalic acid (1 kg, 11.1 mol) was added to isopropyl alcohol (1700 mL) under stirring in a 5 L laboratory reactor. A clear solution formed. Subsequently, p-toluenesulfonic acid monohydrate (47.67 g, 2.5 mol ) in 200 mL toluene was slowly added to the solution. The reaction mixture was heated up and stirred a reflux for 24 h. The generated water was continuously removed by azeotrope with a Dean-Stark trap to drive the reaction to completion. The reaction mixture was cooled down, neutralized with 500 mL satd. aq. NaHCO3, and partitioned between with 400 mL (2x) toluene and 1 L (2x) water. The combined organic phases were dried with 1 L of satd. aq. NaCl solution. The organic phase was separated, and the solvent was removed under vacuum. The crude material was purified by distillation under high vacuum with heating to obtain 1740 g (90%) diisopropyl oxalate as colorless oil. l3C NMR (100 MHz, CDCl3) δ (ppm) 157.96, 71,44, 21.63 (Figure 1 A); 1H NMR (400 MHz, CDCl3) δ 5.13 (hept, J= 6.3 Hz, 2 H), 1.33 (d, J = 6.2 Hz, 12 H) (Figure 1B).
References:
KEMIN INDUSTRIES, INC. WO2021/102115, 2021, A1 Location in patent:Paragraph 0095-0097
40227-15-4
2 suppliers
inquiry

615-81-6
20 suppliers
inquiry
144-62-7
765 suppliers
$5.00/5g
67-63-0
1579 suppliers
$16.00/25ML
57727-06-7
0 suppliers
inquiry

615-81-6
20 suppliers
inquiry
553-90-2
321 suppliers
$10.00/5g

67-63-0
1579 suppliers
$16.00/25ML

615-81-6
20 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry