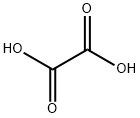
Oxalic acid synthesis
- Product Name:Oxalic acid
- CAS Number:144-62-7
- Molecular formula:C2H2O4
- Molecular Weight:90.03
Nitric acid oxidation is used where carbohydrates, ethylene glycol, and propylene are the starting materials. The dialkyl oxalate process is the newest, where dialkyl oxalate is synthesized from carbon monoxide and alcohol, then hydrolyzed to oxalic acid. This process has been developed by UBE Industries in Japan.Many attempts have been made to synthesize oxalic acid by electrochemical reduction of carbon dioxide in either aqueous or nonaqueous electrolytes.
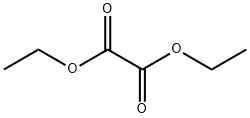
95-92-1
419 suppliers
$5.00/5G

144-62-7
765 suppliers
$5.00/5g
Yield:144-62-7 99.8%
Reaction Conditions:
with ion exchange resin D001 in water at 75; for 1.41667 h;Autoclave;Sealed tube;Large scale;
Steps:
4 Example 4
Ion exchange resin D001 Pretreatment: Take 38.7 kg of deionized water and wash 12.9 kg of ion exchange resin D001 until the discharged water is not yellow. Before use, the ion exchange resin was dried at 85 °C so that the water content of the resin was maintained at 60% by mass.300 kg of deionized water, 10 kg of diethyl oxalate and 13 kg of ion exchange resin were precisely weighed. The deionized water and D001 were weighed into a reaction kettle. The test temperature was set at 75 °C (within ± 0.5 °C), the speed was adjusted to 400 rpm, the beaker filled with diethyl oxalate was sealed, Water bath for preheating.When the temperature of the solution in the autoclave and the temperature of the diethyl oxalate reached 75 °C, the diethyl oxalate was rapidly transferred to the autoclave and the reaction time was recorded simultaneously. During the reaction time sampling, the sample taken into the 10ml volumetric flask,With an electronic balance (accuracy 0.0001)Weighing,Then acetonitrile fixed capacity,And then with an organic filter to filter the sample bottle,The vials were kept in a 0 °C ice-water mixture.The contents of diethyl oxalate and oxalic acid in the samples were determined by high performance liquid chromatography (HPLC).When the reaction time is 85min,The results showed that the oxalate content was 0.0046% Satisfying less than 0.005% Oxalic acid was recovered from the reaction kettle and distilled to separate the oxalic acid. The results of the conversion of dimethyl oxalate / diethyl oxalate and the yield of oxalic acid in each example are shown in Table 1 below.
References:
CN105693502,2016,A Location in patent:Paragraph 0036; 0037; 0038; 0039; 0043
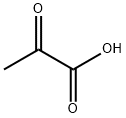
127-17-3
529 suppliers
$5.00/10g

144-62-7
765 suppliers
$5.00/5g
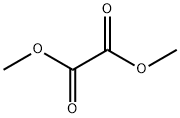
553-90-2
321 suppliers
$10.00/5g

144-62-7
765 suppliers
$5.00/5g
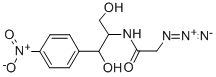
13838-08-9
42 suppliers
$43.00/1mg

144-62-7
765 suppliers
$5.00/5g
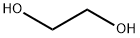
107-21-1
1334 suppliers
$10.00/25g

144-62-7
765 suppliers
$5.00/5g