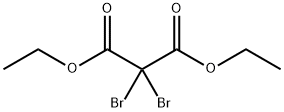
DIETHYL DIBROMOMALONATE synthesis
- Product Name:DIETHYL DIBROMOMALONATE
- CAS Number:631-22-1
- Molecular formula:C7H10Br2O4
- Molecular Weight:317.96

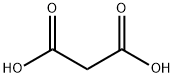
685-87-0
258 suppliers
$10.00/5g

631-22-1
71 suppliers
$20.48/5g
Yield:631-22-1 25%
Reaction Conditions:
Stage #1: Diethyl 2-bromomalonatewith sodium hydride in acetonitrile;mineral oil at 5 - 6; for 2 h;Inert atmosphere;Cooling with ice;
Stage #2: with perfluoroallylfluorosulphate in N,N-dimethyl-formamide;acetonitrile;mineral oil at -35; for 2 h;Inert atmosphere;Solvent;Temperature;
Steps:
4.7. Reaction of diethyl 2-fluoromalonate 1a with perfluoroallyl fluorosulfate (FAFS) 8
General procedure: To a three-neck flask (250 mL) equipped with a thermometer and condenser, a suspension of NaH in mineral oil (60%, 2.71 g. 67.8 mmol, 1.05 equiv.) and dry MeCN (90 mL) were added. The suspension was stirred for 20 min at r. t., then cooled to 8-10 °C and 1a (11.5 g, 64.5 mmol, 10.0 mL) of was added dropwise at this temperature, so that the temperature did not exceed 14 °C. Then, the ice bath was removed, and the reaction mixture was stirred for 2 h until it reached room temperature. Afterwards, the salt solution 2a was poured into a dropping funnel (100 mL) under inert gas atmosphere and the dropping funnel was connected to a three-neck flask (250 mL), which was equipped with a dropping funnel, thermometer and condenser (Method B). In this flask, FAFS 8 (17.1 g, 74.3 mmol, 1.15 equiv.) and dry acetonitrile (75 mL) were added, and the reaction solution was cooled to -15 °C. The solution of 2a was slowly added dropwise at this temperature. During the addition of solution of 2a, a slight exothermic observed. The r. m. was then stirred at -15 °C for 3.5 h, then slowly heated to r. t. and mixed at this temperature for 8 h. The r. m. was evaporated in vacuo and poured into an ice-cold sulfuric acid (2%, 200 mL) and the lower layer was separated. The water phase was extracted with MTBE (3 × 100 mL), the organic phases were combined, washed with saturated NaHCO3 solution (3 × 250 mL) and then dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure. The crude product (18.7 g) was distilled via a short Vigreux column in high vacuum. The olefin 9a was obtained as a yellowish liquid with b. p. 56 - 59 °C / 0.5 mmHg and 72% yield (14.4 g; purity 97%).
References:
Hirschberg, Markus E.;Pajkert, Romana;R?schenthaler, Gerd-Volker;Tverdomed, Sergey N. [Journal of Fluorine Chemistry,2021,vol. 250,art. no. 109864]
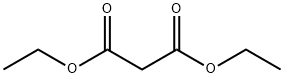
105-53-3
753 suppliers
$5.00/25g

631-22-1
71 suppliers
$20.48/5g
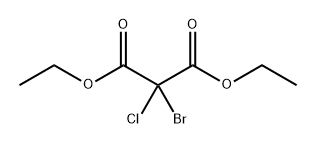
81289-78-3
0 suppliers
inquiry
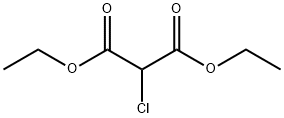
14064-10-9
202 suppliers
$6.00/1g
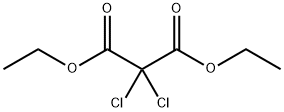
20165-81-5
5 suppliers
inquiry

631-22-1
71 suppliers
$20.48/5g
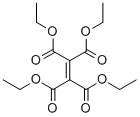
6174-95-4
32 suppliers
$172.00/1g