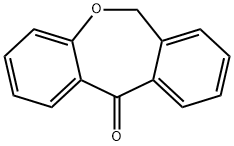
Dibenz[b,e]oxepin-11(6H)-one synthesis
- Product Name:Dibenz[b,e]oxepin-11(6H)-one
- CAS Number:4504-87-4
- Molecular formula:C14H10O2
- Molecular Weight:210.23
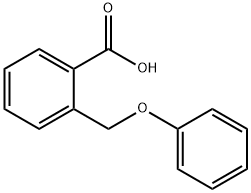
724-98-1
70 suppliers
$25.89/1g
![Dibenz[b,e]oxepin-11(6H)-one](/CAS/GIF/4504-87-4.gif)
4504-87-4
171 suppliers
$5.00/250mg
Yield: 98%
Reaction Conditions:
with trifluoromethylsulfonic anhydride;boron trifluoride diethyl etherate in dichloromethane at 40; for 2 h;
Steps:
237.1 Step 1: dibenzo[b,e]oxepin-11(6H)-one (482)
The 2-(phenoxymethyl)benzoic acid (22.18 g, 97 mmol) was dissolved in DCM (200 mL) and trifluoroacetic anhydride (20.59 mL, 146 mmol) was added, followed by a catalytic amount of borontrifluoride etherate (2.22 mL, 17.5 mmol). The reaction mixture was heated at 40° C. for 2 hours. The solution was then washed with water, sodium bicarbonate (saturated solution) and water. The organic phases was dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified on silica gel (10-20% ethyl acetate in hexanes) to afford title compound 482 (19.937 g, 98%) as a light pink solid. MS (m/z): 211.1 (M+H).
References:
Forum Pharmaceuticals, Inc.;Rogers, Kathryn;Patzke, Holger US2017/749, 2017, A1 Location in patent:Paragraph 0719
87-41-2
469 suppliers
$6.00/10g
![Dibenz[b,e]oxepin-11(6H)-one](/CAS/GIF/4504-87-4.gif)
4504-87-4
171 suppliers
$5.00/250mg
21733-94-8
6 suppliers
inquiry
![Dibenz[b,e]oxepin-11(6H)-one](/CAS/GIF/4504-87-4.gif)
4504-87-4
171 suppliers
$5.00/250mg
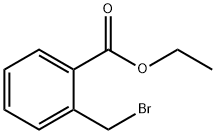
7115-91-5
91 suppliers
$29.00/100mg
![Dibenz[b,e]oxepin-11(6H)-one](/CAS/GIF/4504-87-4.gif)
4504-87-4
171 suppliers
$5.00/250mg
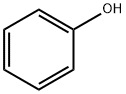
108-95-2
739 suppliers
$14.00/25g
![Dibenz[b,e]oxepin-11(6H)-one](/CAS/GIF/4504-87-4.gif)
4504-87-4
171 suppliers
$5.00/250mg