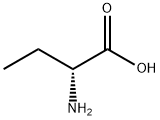
D-2-Aminobutyric acid synthesis
- Product Name:D-2-Aminobutyric acid
- CAS Number:2623-91-8
- Molecular formula:C4H9NO2
- Molecular Weight:103.12
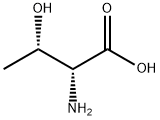
632-20-2
437 suppliers
$6.00/5g

2623-91-8
373 suppliers
$5.00/5g
Yield: 99.9 % ee
Reaction Conditions:
with 2-Oxobutyric acid;(R)-selective omega transaminase;isopropylamine;sodium hydroxide in water; pH=12 for 12 h;Enzymatic reaction;
Steps:
11
L- or D-homo-alanine was synthesized from the passing solution obtained in Example 10 using the omega transaminase purified in Example 4 above.5 N NaOH was added to the passing solution obtained in Example 10 to adjust the pH to 7, and the amount of 2-oxobutyrate in the passing solution was measured by HPLC to be used as a substrate.50 mM 2-oxobutyrate (passage liquid of Example 10 above), 100 mM isopropylamine, 50 mM Phosphate buffer pH 7, 5 mg / mL of (S) -selective omega transaminase from Ochrobactrumthropi at 37 ° C Arthrobacter sp.(R) -elective omega transaminase (6 mg / mL) was added to determine the amount of homo-alanine over time, which was divided into L-homo-alanine and D-homo-alanine,Its enantiomeric excess (ee) was determined.As a result, L-homo-alanine was obtained with a conversion rate of 93.2% and enatiomeric excess of 99.9% or more only after 9 hours of reaction with (S) -selective omega transaminase,(R) - selective omega transaminase, D-homo-alanine was obtained with a conversion rate of 89.4% and an enatiomeric excess of 99.9% or more in only 12 hours of reaction.
References:
Yonsei University Industry-Academic Cooperation Foundation,;Sin, Jong Sik;Han, Sang Woo KR2016/96906, 2016, A Location in patent:Paragraph 0058-0059
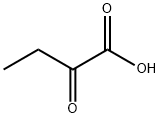
600-18-0
260 suppliers
$23.00/1g

2623-91-8
373 suppliers
$5.00/5g
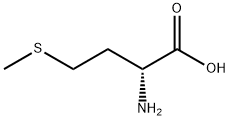
348-67-4
381 suppliers
$5.00/1g

2623-91-8
373 suppliers
$5.00/5g
1492-24-6
573 suppliers
$6.00/10g

2623-91-8
373 suppliers
$5.00/5g