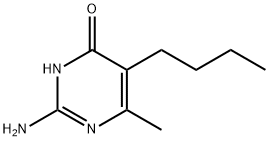
CHEMBRDG-BB 9072033 synthesis
- Product Name:CHEMBRDG-BB 9072033
- CAS Number:4038-64-6
- Molecular formula:C9H15N3O
- Molecular Weight:181.23
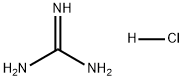
50-01-1
789 suppliers
$5.00/25g
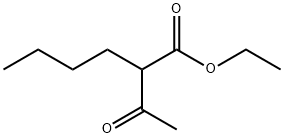
1540-29-0
147 suppliers
$15.00/5mL

4038-64-6
22 suppliers
$35.35/1mg:
Yield:4038-64-6 16.86 g
Reaction Conditions:
Stage #1: guanidine hydrochloridewith sodium methylate in methanol at 20;
Stage #2: ethyl 2-butylacetoacetate in methanol;Reflux;
Steps:
1.1; 2.1; 3.1; 4.1; 5.1
Step 1, at ambient temperature, sequentially add guanidine hydrochloride (14.33g), methanol (70mL), and magnetons to the reflux condenser,In the 250mL three-necked round-bottomed flask of thermometer, open and stir under ambient temperature, use solid addition funnel, divide into three batches on average, add sodium methylate solid (8.10g) in the system at intervals of 20 minutes,After the addition is complete, continue to stir at ambient temperature for 1 hour, add ethyl 2-n-butylacetoacetate (18.63 g) once to the system, turn on the heating, and raise the temperature of the system to solvent reflux.Take a sample TLC plate to monitor the disappearance of ethyl 2-n-butylacetoacetate and stop heating. After the system temperature drops to ambient temperature, add 5% dilute hydrochloric acid to the system to neutralize to PH = 7, and use a rotary evaporator to distill methanol Afterwards, a large amount of white solids were separated out, and 70 mL of water was added to the system,After stirring and beating for 30 minutes at ambient temperature, filter, rinse with 45mL water for three times, and put the solid in a vacuum oven at 70°C to dry.16.86 g of white solid was obtained, which was 2-amino-5-n-butyl-6-methyl-4-hydroxypyrimidine, with a content of 98.5%.
References:
CN115403527,2022,A Location in patent:Paragraph 0037; 0057-0060; 0064-0066; 0069-0071; 0074; ...