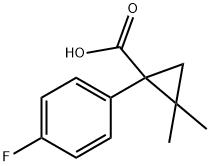
CHEMBRDG-BB 4006362 synthesis
- Product Name:CHEMBRDG-BB 4006362
- CAS Number:915921-31-2
- Molecular formula:C12H13FO2
- Molecular Weight:208.23
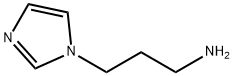
5036-48-6
294 suppliers
$14.00/1g

915921-31-2
15 suppliers
$120.00/200μmol
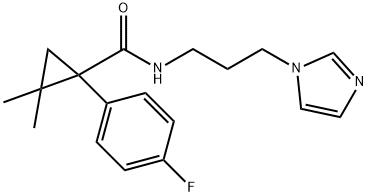
1434927-54-4
9 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: C12H13FO2with 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 24 h;
Stage #2: 3-(1H-imidazol-1-yl)propan-1-amine in tetrahydrofuran; for 48 h;
Steps:
Synthesis of C10d and derivatives
General procedure: An appropriate substituted benzoic acid side chain (0.0023 mmol) was activated using CDI (0.0023 mmol) in THF for 24 hours at room temperature. 1-(3-aminopropyl)imidazole (0.0023 mm0l) was added dropwise upon stirring for 48 hours. Solvent was removed under reduced vacuum, and extracted with 4x50 ml of DCM, washed with water 3x100 mL and dried with magnesium sulfate. DCM was then removed under vacuum to yield the specific compounds. Synthesis of lead compounds was confirmed via 1H NMR, mass spectrometry. Instrumentation: 1H NMR was collected on a 300 MHz Varian Mercury spectrometer, and mass spectrometry was done using a Waters Mass spectrometer. Compound C10d. 1H NMR (300 MHz, CD3OD) δH 7.68 (s, 1H), 7.57 (dd, J = 8.6, 5.4 Hz, 2H), 7.52 (s, 1H), 7.46 (dd, J = 8.5, 5.6 Hz, 2H), 6.92 (s, 1H), 3.88 (t, J = 7.0 Hz, 2H), 3.09 (t, J = 6.7 Hz, 2H), 1.88 (quin, J = 6.8 Hz, 2H), 1.58 (dd, J = 28.7, 5.6 Hz, 2H), 0.86 (s, 6H). MS [M + H]+ calculated for C18H22FN3O: 316.1825, found: 316.1792.
References:
Geldenhuys, Werner J.;Caporoso, Joel;Leeper, Thomas C.;Lee, Yoon-Kwang;Lin, Li;Darvesh, Altaf S.;Sadana, Prabodh [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 2,p. 303 - 308] Location in patent:supporting information