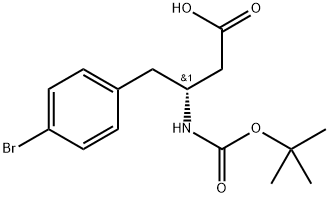
BOC-(R)-3-AMINO-4-(4-BROMO-PHENYL)-BUTYRIC ACID synthesis
- Product Name:BOC-(R)-3-AMINO-4-(4-BROMO-PHENYL)-BUTYRIC ACID
- CAS Number:331763-75-8
- Molecular formula:C15H20BrNO4
- Molecular Weight:358.23

331763-75-8
90 suppliers
$33.00/100mg
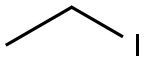
75-03-6
362 suppliers
$11.00/5g
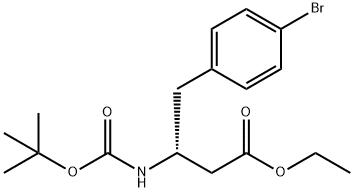
1257446-29-9
4 suppliers
inquiry
Yield:1257446-29-9 94%
Reaction Conditions:
with sodium hydrogencarbonate in N,N-dimethyl-formamide at 20; for 71 h;Inert atmosphere;
Steps:
5
Intermediate 5: (R)-ethyl 4-(4-bromophenyi)-3-(tert-butoxycarbonyfamino)butanoate; To a suspension of (R)-4-(4~brornophenyl)-3-(tert-butoxycarbonylamino)butanoic acid (9.98 g, 27,9 mmoi) and NaHCO3 (4.68 g, 55.7 mmol) in OMF (45 mL) is added Ethyl iodide (6.75 mL, 84 mmol) at room temperature under nitrogen. After stirring for 71 hours, the reaction is quenched with H2O (300 mL), and then precipitated solid is collected and washed with H2O (500 mL) to give (R)-ethyl 4-(4-bromophenyl)-3~(tert-butoxycarbonylamino)butanoate (10.25 g, 94%). HPLC retention time ? 1.48 minutes (condition B); MS (ES+) = 329.9 (m-tBu+2); 286,0 (m-Boc+2; 100%); 1H NMR (400 MHz1 CHLOROFORM-cQ δ ppm 1.27 (t, J = 7.2 Hz, 3 H) 1.40 (S1 9 H)1 2.43 (A Of ABX1 Jab ? 15.8 Hz, Jax * 5.7 Hz1 1 H) 2.50 (B of ABX, Jab = 15.8 Hz, Jbx - 5.4 Hz, 1 H) 2.74 - 2.90 (m, 2 H) 4.11 (br 8) 4.15 (q, J = 7.1 Hz, 2 H) 5,04 (br d) 7,07 (d, J = 8.3 Hz1 2 H) 7.40-7.43 (m, 2 H).
References:
WO2010/136493,2010,A1 Location in patent:Page/Page column 206

331763-75-8
90 suppliers
$33.00/100mg
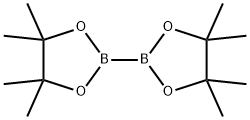
73183-34-3
578 suppliers
$6.00/5g
![Benzenebutanoic acid, β-[[(1,1-dimethylethoxy)carbonyl]amino]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, (βR)-](/CAS/20210305/GIF/1246361-96-5.gif)
1246361-96-5
0 suppliers
inquiry