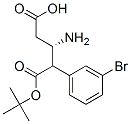
Boc-(S)-3-Amino-4-(3-bromo-phenyl)-butyric acid synthesis
- Product Name:Boc-(S)-3-Amino-4-(3-bromo-phenyl)-butyric acid
- CAS Number:919988-44-6
- Molecular formula:C15H20BrNO4
- Molecular Weight:358.23
919988-42-4
11 suppliers
inquiry

24424-99-5
833 suppliers
$13.50/25G

919988-44-6
24 suppliers
inquiry
Yield:919988-44-6 56%
Reaction Conditions:
Stage #1: 3-amino-4-(3-bromophenyl)butanoic acid;di-tert-butyl dicarbonatewith sodium hydroxide in methanol;water at 20; for 2 h;
Stage #2: with hydrogenchloride in methanol;water; pH=3;
Steps:
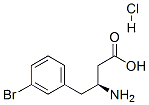
1A 4-(3-Bromophenyl)-3-[(tert-butoxycarbonyl)amino]butanoic acid
62 ml of a 1N sodium hydroxide solution are added to a solution of 8.0 g (31.1 mmol) of 3-amino-4-(3-bromophenyl)butanoic acid in 100 ml of water. While stirring, a solution of 20 g (93 mmol) of di-tert-butyl dicarbonate in 100 ml of methanol is added thereto at RT, and the mixture is stirred for 2 h. The pH is adjusted to 3 by adding 0.1N hydrochloric acid, and the mixture is extracted twice with ethyl acetate. The organic phases are combined, dried with magnesium sulfate and evaporated to dryness in vacuo. The remaining solid is used without further purification.Yield: 7.0 g (56% of theory)LC-MS (Method 3): Rt=2.55 minMS (EI): m/z=359 (M+H)+
References:
US2008/306040,2008,A1 Location in patent:Page/Page column 11-12