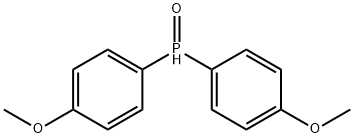
Bis(4-methoxyphenyl)phosphine oxide synthesis
- Product Name:Bis(4-methoxyphenyl)phosphine oxide
- CAS Number:15754-51-5
- Molecular formula:C14H15O3P
- Molecular Weight:262.24
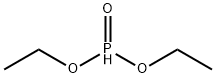
762-04-9
326 suppliers
$10.00/10 g
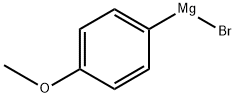
13139-86-1
132 suppliers
$45.00/5g

15754-51-5
27 suppliers
inquiry
Yield: 92%
Reaction Conditions:
Stage #1:phosphonic acid diethyl ester;4-methoxyphenyl magnesium bromide in tetrahydrofuran at 0 - 20; for 2.33333 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;tert-butyl methyl ether;water at 0; for 0.416667 h;
Steps:
General procedure A: symmetric secondary phosphine oxides Bis-(4-methoxyphenyl)-phosphine oxide
Symmetric secondary phosphine oxides were prepared by the method of Hays by adding 1 eq. of diethylphosphite to 3.3 eq. of the corresponding Grignard reagent at 0 °C and warming to ambient temperature. A 100-mL round-bottom flask equipped with an addition funnel was evacuated/Ar filled, then charged with 33 mL of 1 M 4-methoxyphenylmagnesium bromide/THF (33 mmol, 3.3 eq.), and the solution cooled to 0 °C under Ar. A solution of 1.38 g of diethylphosphite (10 mmol, 1.0 eq.) in 10 mL of THF was then added dropwise over 10 min. The mixture was aged 10 min at 0 °C, then the bath was removed, and the mixture stirred 2 h at ambient temperature, then cooled again to 0 °C. 25 mL 0.1 N HCl (deoxygenated water) was then added dropwise over 20 min, then 25 mL methyl tert-butyl ether (MTBE) was added, and the mixture agitated well for 5 min. The upper organic phase was decanted from the separator and saved. To the lower phase was then added 20 mL of 1 N HCl (deoxygenated water) again, and it was extracted with CH2Cl2 (2 9 30 mL). The organic phase was combined with the first organic phase, then filtered through a celite pad, washing the pad with CH2Cl2, dried magnesium sulfate (MgSO4), and the solvents removed in vacuo. The crude material was chromatographed on silica gel eluting with 2% MeOH/CH2Cl2, then recrystallized from hot ethyl acetate (EtOAc) to give 2.41 g of product (92%) as a colorless solid. Colorless solid; YIELD = 92%; 1H NMR (400 MHz, CDCl3) d: 8.01 (d, 1JHP = 473 Hz, 1H), 7.60 (dd, J = 13.8, 8.2 Hz, 4H), 6.97 (dd, J = 7.8, 2.4 Hz, 4H), 3.83 (s, 6H); 31P NMR (162 MHz, CDCl3) d: 21.0.
References:
Li, Chun-Jing;Lü, Jing;Zhang, Zhi-Xun;Zhou, Kun;Li, Yan;Qi, Guang-Hui [Research on Chemical Intermediates,2018,vol. 44,# 7,p. 4547 - 4562]
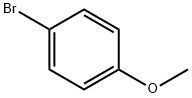
104-92-7
428 suppliers
$10.00/10g

15754-51-5
27 suppliers
inquiry

104-92-7
428 suppliers
$10.00/10g

762-04-9
326 suppliers
$10.00/10 g

15754-51-5
27 suppliers
inquiry

13139-86-1
132 suppliers
$45.00/5g

15754-51-5
27 suppliers
inquiry
803-17-8
1 suppliers
inquiry

15754-51-5
27 suppliers
inquiry