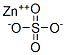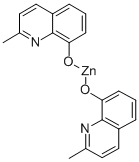
BIS(2-METHYL-8-HYDROXYQUINOLINATO)ZINC synthesis
- Product Name:BIS(2-METHYL-8-HYDROXYQUINOLINATO)ZINC
- CAS Number:14128-73-5
- Molecular formula:C20H16N2O2Zn
- Molecular Weight:381.748
Yield:14128-73-5 80%
Reaction Conditions:
with tetrabutylammonium perchlorate in acetonitrile; for 1 h;Electrochemical reaction;
Steps:
Preparation of [Zn(Mq)2] by the Electrochemical Method:
The electrochemical procedure followed in the synthesis ofcomplex was similar to that described by Habeeb and Tuck[21]. In a 100 cm3 tall-form beaker, through which the electrochemicalleads (Zinc as the anode and platinum as the cathode) entered, electrochemical oxidation of the Zinc anodein a solution of 2-methyl-8-hydroxyquinoline (0.1591 g,1.0 mmol) in acetonitrile (50 ml) containing 10 mg oftetrabutylammonium perchlorate as supporting electrolyte at30 mA for 1 h caused 47 mg of Zinc to be dissolved,EF = 0.78 mol/F. This cell can be summarized as: Pt(-)|HL + MeCN |Zn(+), and the synthesis is identified by thepreparation of ZnL2. During electrolysis hydrogen wasevolved at the cathode and at the end of the experiment theyellow solid was filtered, washed with acetonitrile and diethylether and dried under vacuum. The complex was fully characterizedbased on its 1HNMR, 13C NMR and IR spectra andelemental analysis. (yield 80%, m.p > 270 °C). Anal. Calc. forC20H16N2O2Zn: C, 60.1; H, 4.5; N, 7.0%. Found: C, 59.7; H,4.5; N, 6.9%. IR (KBr, cm-1): 3046(w), 2983(m), 1548(m),1495(w), 1332(w), 465(m). 1H NMR (DMSO-d6): 2.92(s,6H), 6.43, 6.88, 7.25, 7.45 and 8.25 (m, 10H, Ph-H). 13CNMR (DMSO, d6): 24.3 (CH3), 110.4, 116.6, 123.5, 128.1,134.4, 139.1, 140.2, 155.8, 162.5 (Ar-C).
References:
Jodaian, Vida;Shahrjerdi, Akram;Mirahmadpour, Pari [Journal of Fluorescence,2017,vol. 27,# 2,p. 715 - 722]
95-55-6
541 suppliers
$5.00/5G

14128-73-5
16 suppliers
inquiry
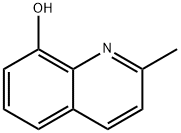
826-81-3
274 suppliers
$11.00/25g
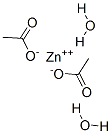
5970-45-6
353 suppliers
$13.50/25G

14128-73-5
16 suppliers
inquiry

826-81-3
274 suppliers
$11.00/25g
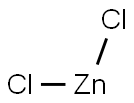
7646-85-7
562 suppliers
$10.00/25ml

14128-73-5
16 suppliers
inquiry