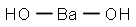
Barium hydroxide synthesis
- Product Name:Barium hydroxide
- CAS Number:17194-00-2
- Molecular formula:BaH2O2
- Molecular Weight:171.34
BaS + 2NaOH → Ba(OH)2 + Na2S.
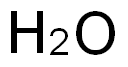
7732-18-5
490 suppliers
$12.69/100ml
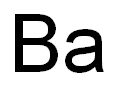
7440-39-3
149 suppliers
$39.50/50ml
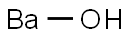
12009-08-4
2 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
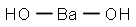
17194-00-2
313 suppliers
$14.00/5g
Yield:-
Reaction Conditions:
in solid matrixIrradiation (UV/VIS); heating of water, Ar (excess) and Ca at 465 - 550°C for 1 or 2 hin steel crucible in Ta furnace and cocondensation on mirrored Cu held at 15 K; photolysis of formed matrices with Xe or Hg lamp for 15 min to 2.5 h; reaction mechanisms described; not isolated; detn. by IR; mixture of HBaOH, Ba(OH)2 and Ba(OH) obtained;
References:
Kauffman;Hauge;Margrave [High temperature science,1984,vol. 18,# 2,p. 97 - 118]