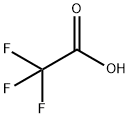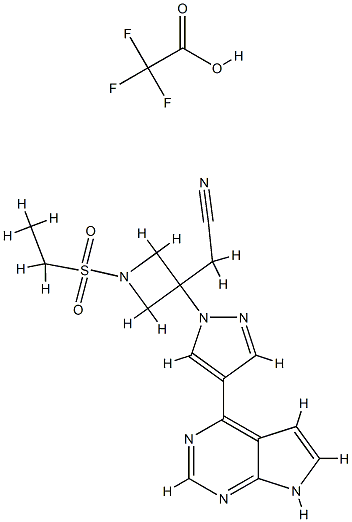
Baricitinib trifluoroacetate synthesis
- Product Name:Baricitinib trifluoroacetate
- CAS Number:1187594-10-0
- Molecular formula:C18H18F3N7O4S
- Molecular Weight:485.4402
Yield:1187594-10-0 47%
Reaction Conditions:
Stage #1: 2-[1-ethanesulfonyl-3-[4-(7-[(2-(trimethylsilyl)ethoxy)methyl]-7H-pyrrolo[2,3-d]pyrimidine-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl]acetonitrilewith trifluoroacetic acid in dichloromethane; for 1.5 h;
Stage #2: with ethylenediamine in methanol; for 3 h;
Stage #3: trifluoroacetic acid in water;acetonitrile;
Steps:
1.5
To a solution of 1-(ethylsulfonyl)-3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-ylacetonitrile (0.111 g, 0.22 mmol) in methylene chloride (3 mL) was added trifluoroacetic acid (2 mL) and the solution was stirred for 1.5 hours. The solvents were removed in vacuo and the residue was dissolved in methanol (3 mL) and ethylenediamine (0.1 mL) was added. After stirring for 3 hours, the volume was reduced in vacuo and the product was purified by preparative-HPLC/MS, (SunFire C18 column, eluding with a gradient of MeCN/H2O containing 0.1% TFA) to afford the product as the trifluoroacetic acid salt (50 mg, 47%). 1H NMR (400 MHz, d6-dmso): δ 12.55 (br d, 1H), 9.03 (s, 1H), 8.83 (s, 1H), 8.56 (s, 1H), 7.79-7.75 (m, 1H), 7.24-7.19 (m, 1H), 4.59 (d, 2H), 4.26 (d, 2H), 3.71 (s, 2H), 3.25 (q, 2H), 1.24 (t, 3H); LCMS (M+H)+: 372.1.
References:
US2009/233903,2009,A1 Location in patent:Page/Page column 28
![2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile](/CAS/20180531/GIF/1187594-13-3.gif)