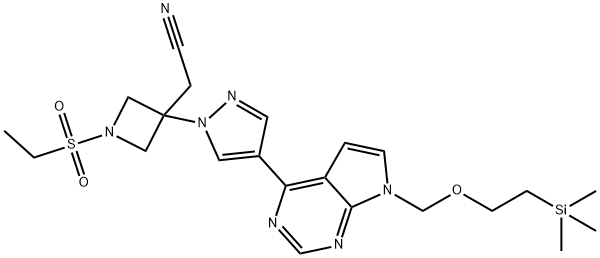
2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile synthesis
- Product Name:2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile
- CAS Number:1187594-13-3
- Molecular formula:C22H31N7O3SSi
- Molecular Weight:501.68
![3-Azetidineacetonitrile, 3-[4-[7-[[2-(trimethylsilyl)ethoxy]methyl]-7H-pyrrolo[2,3-d]pyrimidin-4-yl]-1H-pyrazol-1-yl]-](/CAS/20210305/GIF/1187594-12-2.gif)
1187594-12-2
1 suppliers
inquiry
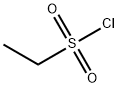
594-44-5
253 suppliers
$21.00/25g
![2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile](/CAS/20180531/GIF/1187594-13-3.gif)
1187594-13-3
54 suppliers
inquiry
Yield:1187594-13-3 91%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran; for 1.5 h;Product distribution / selectivity;
Steps:
1.4
To a solution of 3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-ylacetonitrile (0.100 g, 0.244 mmol) in tetrahydrofuran (2 mL) containing N,N-diisopropylethylamine (0.085 mL, 0.49 mmol) was added ethanesulfonyl chloride (0.023 mL, 0.24 mmol). After stirring for 1.5 hours, the reaction mixture was poured into dilute HCl and extracted with three portions of ethyl acetate. The combined extracts were washed with brine, dried over sodium sulfate, decanted and concentrated to afford product, used without further purification in Step 5 (111 mg, 91%). 1H NMR (300 MHz, CDCl3): δ 8.86 (s, 1H), 8.63 (s, 1H), 8.35 (s, 1H), 7.45 (d, 1H), 6.83 (d, 1H), 5.68 (s, 2H), 4.63 (d, 2H), 4.26 (d, 2H), 3.54 (t, 2H), 3.42 (s, 2H), 3.09 (q, 2H), 1.41 (t, 3H), 0.92 (t, 2H), -0.06 (s, 9H); LCMS (M+H)+: 502.1.
References:
US2009/233903,2009,A1 Location in patent:Page/Page column 27-28
![4-(1H-Pyrazol-4-yl)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/941685-27-4.gif)
941685-27-4
239 suppliers
$18.00/250mg
1187595-85-2
266 suppliers
inquiry
![2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile](/CAS/20180531/GIF/1187594-13-3.gif)
1187594-13-3
54 suppliers
inquiry

1187595-85-2
266 suppliers
inquiry
![3-Azetidineacetonitrile, 3-[4-[7-[[2-(trimethylsilyl)ethoxy]methyl]-7H-pyrrolo[2,3-d]pyrimidin-4-yl]-1H-pyrazol-1-yl]-](/CAS/20210305/GIF/1187594-12-2.gif)
1187594-12-2
1 suppliers
inquiry
1187595-86-3
1 suppliers
inquiry
![2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile](/CAS/20180531/GIF/1187594-13-3.gif)
1187594-13-3
54 suppliers
inquiry
![4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/941685-26-3.gif)
941685-26-3
178 suppliers
$59.00/250mg
![2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile](/CAS/20180531/GIF/1187594-13-3.gif)
1187594-13-3
54 suppliers
inquiry
76513-69-4
356 suppliers
$6.00/1g
![2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile](/CAS/20180531/GIF/1187594-13-3.gif)
1187594-13-3
54 suppliers
inquiry