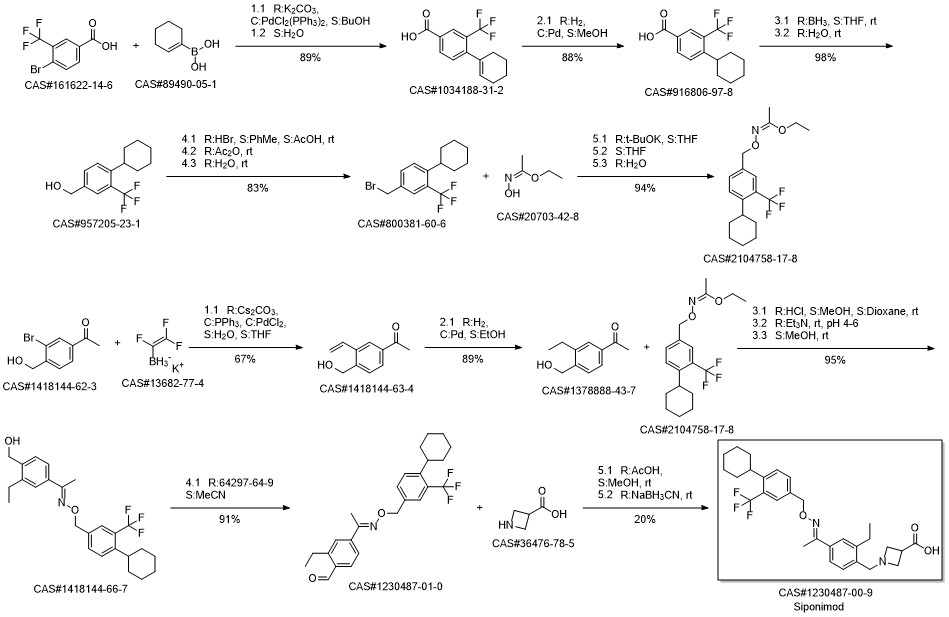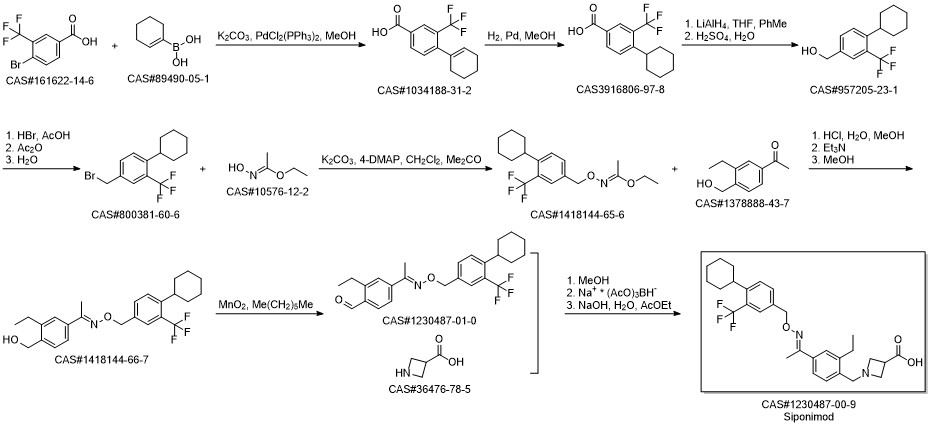
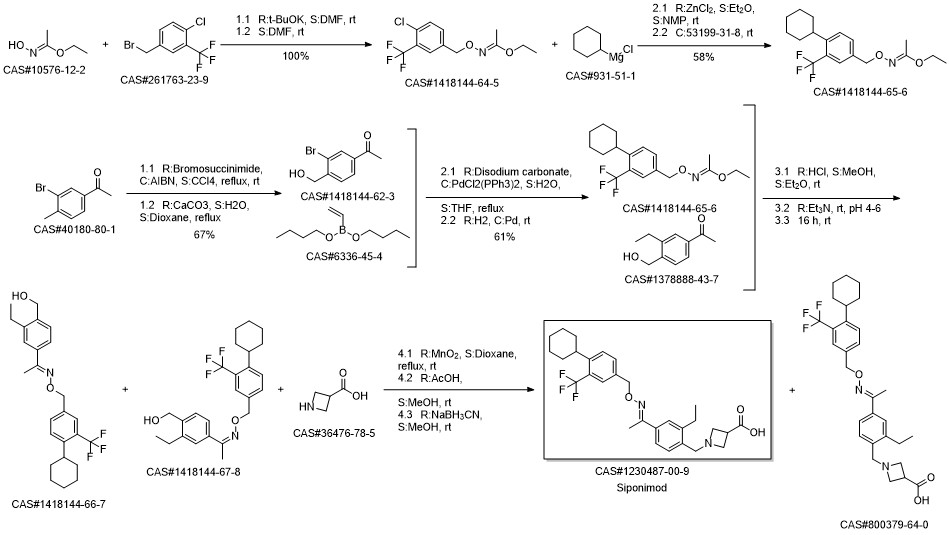
BAF-312(SiponiMod) synthesis
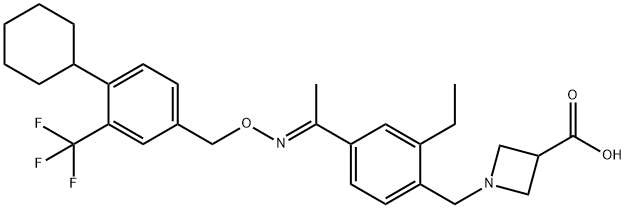
- Product Name:BAF-312(SiponiMod)
- CAS Number:1230487-00-9
- Molecular formula:C29H35F3N2O3
- Molecular Weight:516.59
Reference: Pan, Shifeng; Gray, Nathanael S.; Gao, Wenqi; Mi, Yuan; Fan, Yi; Wang, Xing; Tuntland, Tove; Che, Jianwei; Lefebvre, Sophie; Chen, Yu; Chu, Alan; Hinterding, Klaus; Gardin, Anne; End, Peter; Heining, Peter; Bruns, Christian; Cooke, Nigel G.; Nuesslein-Hildesheim, Barbara. Discovery of BAF312 (Siponimod), a Potent and Selective S1P Receptor Modulator. ACS Medicinal Chemistry Letters. Volume 4. Issue 3. Pages 333-337. Journal; Online Computer File. (2013).
![Benzaldehyde, 4-[(1E)-1-[[[4-cyclohexyl-3-(trifluoromethyl)phenyl]methoxy]imino]ethyl]-2-ethyl-](/CAS/20180703/GIF/1230487-01-0.gif)
1230487-01-0
15 suppliers
inquiry
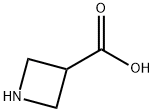
36476-78-5
429 suppliers
$6.00/1g

1230487-00-9
159 suppliers
$28.00/1mg
Yield:1230487-00-9 89%
Reaction Conditions:
Stage #1: 4-[(1E)-1-([[4-cyclohexyl-3-(trifluoromethyl)phenyl]methoxy]imino)ethyl]-2-ethylbenzaldehyde;3-azetidinecarboxylic acid in methanol at 20 - 25; for 0.5 h;
Stage #2: with methanol;sodium tris(acetoxy)borohydride
Steps:
1 Example 1 : Preparation of amorphous form of compound of formula (I)
26 g of (E)-4-(l-(((4-cyclohexyl-3-(trifluoromethyl)benzyl)oxy)imino)ethyl)-2- ethylbenzaldehyde and 8.53 g of azetidine-3 -carboxylic acid were suspended in 400 ml of MeOH at 20°C-25°C. The suspension was stirred at 20°C-25°C for 30 minutes. 24.3 g of Sodium triacetoxyborohydride was added in 8 equal portions in 10-15 min intervals. The reaction was finished right after the final portion of the reducing agent was added. The solvent was removed by evaporation and the residue was diluted with 156 ml of water and 312 ml of ethyl acetate. pH of the mixture was adjusted by addition of 75 ml of 2M aqueous NaOH to approx 6 at 20°C-25°C. The phases were separated and the organic phase was washed with 60 ml of water, dried using MgS04 and filtered. To the filtrate was added 170 ml of absolute EtOH , the solution was concentrated to the of the original volume. The mixture was again dried using MgSCH, filtered and concentrated to amount of 140 g. 170 ml of absolute EtOH absolute was added and the mixture was concentrated to amount of 140 g. 170 ml of absolute EtOH and 2.4 g of activated charcoal were added and the suspension was agitated for 30 min. The suspension was filtered and the filtrate was concentrated to amount 50 g. To this oily residue, 100 ml of n-heptane was added and the solution was again concentrated to amount 50 g. 100 ml of n-heptane was added and the solution was concentrated to dryness. The formed foam was dried to provide foam that can be crushed to white solid amorphous compound of formula (I), 28.22 g (89% of theoretical yield), 98.5% purity (HPLC in).
References:
WO2020/208167,2020,A1 Location in patent:Page/Page column 14; 15
![Ethanone, 1-[3-ethyl-4-(hydroxymethyl)phenyl]-,O-[[4-cyclohexyl-3-(trifluoromethyl)phenyl]methyl]oxime, (1E)-](/CAS/20180703/GIF/1418144-66-7.gif)
1418144-66-7
8 suppliers
inquiry

36476-78-5
429 suppliers
$6.00/1g

1230487-00-9
159 suppliers
$28.00/1mg
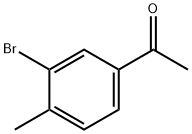
40180-80-1
188 suppliers
$23.00/1g

1230487-00-9
159 suppliers
$28.00/1mg
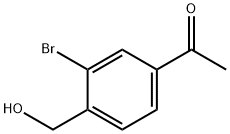
1418144-62-3
18 suppliers
inquiry

1230487-00-9
159 suppliers
$28.00/1mg
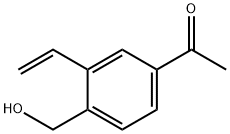
1418144-63-4
7 suppliers
inquiry

1230487-00-9
159 suppliers
$28.00/1mg