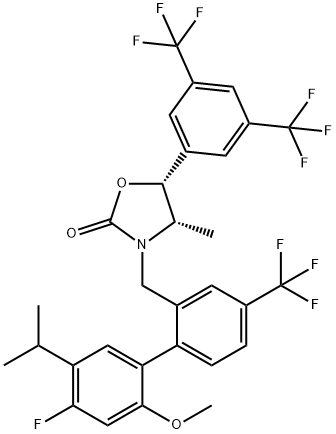
Anacetrapib synthesis
- Product Name:Anacetrapib
- CAS Number:875446-37-0
- Molecular formula:C30H25F10NO3
- Molecular Weight:637.51
![2-Oxazolidinone, 5-[3,5-bis(trifluoromethyl)phenyl]-4-methyl-, (4S,5R)-](/CAS/20180906/GIF/875444-08-9.gif)
875444-08-9
131 suppliers
$55.00/250mg

875548-97-3
53 suppliers
$327.00/1g

875446-37-0
188 suppliers
$15.00/1mg
Yield:875446-37-0 72%
Reaction Conditions:
Stage #1: (4'-fluoro-5'-isopropyl-2'-methoxy-4-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)methanolwith thionyl chloride in dichloromethane at 5 - 20;
Stage #2: (4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-4-methyl-1,3-oxazolidin-2-onewith tetra-(n-butyl)ammonium iodide;potassium carbonate in dichloromethane at 60;
Steps:
1.3
Take 100ml round mouth flask, add intermediate V and methylene chloride, cool to below 5 , stirring slowly dropping thionyl chloride.The resulting reaction mixture was incubated at 15-20 & lt; 0 & gt; C for 2-3 hours and the TLC monitoring reaction was complete.The mixture was stirred with water for 10 minutes, and the layers were allowed to stand apart. The aqueous layer was removed and compound VI was added to a 100 ml round bottom flask followed by tetrabutylammonium iodide, K2CO3, And the mixture was stirred at 60 ° C for 16-18 hours. TLC was used to monitor the reaction. After completion of the reaction, n-heptane and water were added under heat.The aqueous layer was discarded and the organic layer was washed with water (3 times) and concentrated to give a yellow liquid, which was added with ethanol: water (1: 20 by volume) and allowed to stand to give a white powder. The final product Compound).Yield 72%.
References:
CN106032362,2016,A Location in patent:Paragraph 0045-0048
![2-Oxazolidinone, 5-[3,5-bis(trifluoroMethyl)phenyl]-3-[[2-iodo-5-(trifluoroMethyl)phenyl]Methyl]-4-Methyl-, (4S,5R)-](/CAS/20150408/GIF/875446-35-8.gif)
875446-35-8
1 suppliers
inquiry
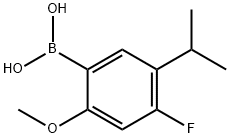
875446-29-0
149 suppliers
$30.00/1g

875446-37-0
188 suppliers
$15.00/1mg
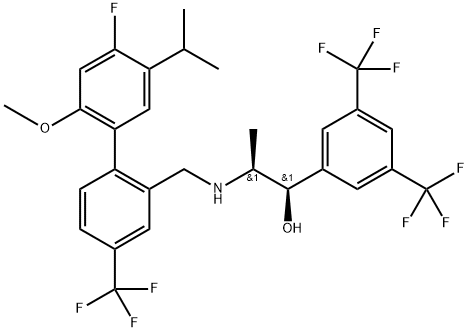
1220703-07-0
1 suppliers
inquiry
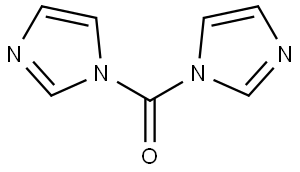
530-62-1
936 suppliers
$6.00/25g

875446-37-0
188 suppliers
$15.00/1mg
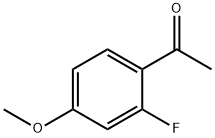
74457-86-6
258 suppliers
$6.00/5g

875446-37-0
188 suppliers
$15.00/1mg
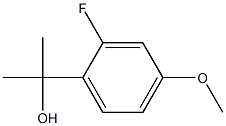
96826-25-4
7 suppliers
inquiry

875446-37-0
188 suppliers
$15.00/1mg