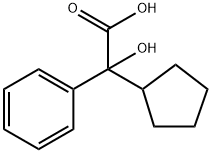
alpha-Cyclopentylmandelic acid synthesis
- Product Name:alpha-Cyclopentylmandelic acid
- CAS Number:427-49-6
- Molecular formula:C13H16O3
- Molecular Weight:220.27
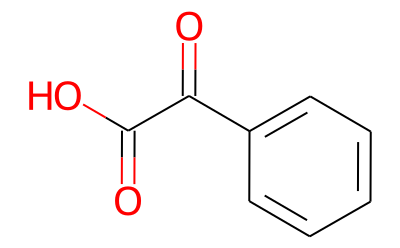
611-73-4
293 suppliers
$36.00/5g
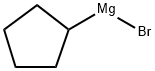
33240-34-5
187 suppliers
$88.00/100g

427-49-6
317 suppliers
$6.00/1g
Yield:427-49-6 36.4%
Reaction Conditions:
in diethyl ether at 0 - 20; for 24.5 h;
Steps:
Cyclopentylmagnesium bromide ether solution (100 ml, 2M; 0.2 mol) was added drop-wise to benzoylformic acid (15 g, 0.1 mol) in 330 ml of anhydrous ethyl ether at 0° C. The mixture was stirred at 0° C. for 30 min and at room temperature for 24 h. The reaction mixture was treated with 1 N HCl, and the aqueous solution was extracted with ether. The combined ether solution was treated with K2CO3 solution. The potassium carbonate solution was acidified with HCl and extracted with ether twice. The ether solution was dried with anhydrous sodium sulfate and evaporated to give a crude product. The crude product was washed with water to get pure racemic cyclopentylmandelic acid 1 (8.0 g, 36.4%). Needle-like crystal, m.p.: 153-154° C. 1H NMR (CDCl3,300 MHz): 1.28-1.39, 1.42-1.50, 1.51-1.61, 1.63-1.72 [8H, m, (CH2)4], 2.93 [1H, p, CHC(OH)], 7.26-7.30, 7.33-7.36, 7.65-7.67(5H, m, Ph) ppm.; Cyclopentylmagnesium bromide ether solution (100 ml, 2M; 0.2 mol) was added drop-wise to benzoylformic acid (15 g, 0.1 mol) in 330 ml of anhydrous ethyl ether at 0° C. The mixture was stirred at 0° C. for 30 min and at room temperature for 24 h. The reaction mixture was treated with 1 N HCl, and the aqueous solution was extracted with ether. The combined ether solution was treated with K2CO3 solution. The potassium carbonate solution was acidified with HCl and extracted with ether twice. The ether solution was dried with anhydrous sodium sulfate and evaporated to give a crude product. The crude product was washed with water to get pure racemic cyclopentylmandelic acid 1 (8.0 g, 36.4%). Needle-like crystals, m.p.: 153-154° C. 1H NMR (CDCl3, 500 MHz): 1.28-1.39, 1.42-1.50, 1.51-1.61, 1.63-1.72 [8H, m, (CH2)4], 2.93 [1H, p, CHC(OH)], 7.26-7.30, 7.33-7.36, 7.65-7.67(5H, m, Ph) ppm.
References:
Bodor, Nicholas S. US2007/123557, 2007, A1 Location in patent:Page/Page column 9; 12; 15-16
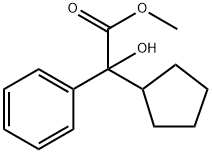
19833-96-6
194 suppliers
$17.00/1g

427-49-6
317 suppliers
$6.00/1g
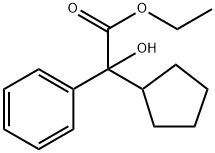
16098-80-9
12 suppliers
inquiry

427-49-6
317 suppliers
$6.00/1g
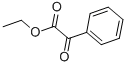
1603-79-8
205 suppliers
$7.00/5g

427-49-6
317 suppliers
$6.00/1g

33240-34-5
187 suppliers
$88.00/100g

427-49-6
317 suppliers
$6.00/1g