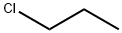
1-Chloropropane synthesis
- Product Name:1-Chloropropane
- CAS Number:540-54-5
- Molecular formula:C3H7Cl
- Molecular Weight:78.54

74-98-6
125 suppliers
$20.00/1ea
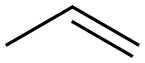
187737-37-7
1 suppliers
inquiry
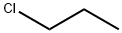
540-54-5
223 suppliers
$18.00/25mL
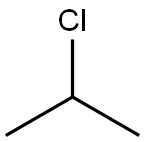
75-29-6
329 suppliers
$19.00/25mL
Yield:-
Reaction Conditions:
with chlorine at 350; under 760.051 Torr;Flow reactor;Gas phase;Darkness;Reagent/catalyst;Temperature;
Steps:
Catalytic Tests
General procedure: Catalytic experiments were carried out at atmospheric pressure in a tubular flow reactor (quartz tube200 mm in length and 7 mm in inner diameter) with afixed catalyst bed. The reactor was heated in a tubularelectric furnace to 150-450°C. The weight of the catalyst placed in the reactor was 1.0 g. Glassfiber samples, which were loose balls of fibers, were compactedto the minimum possible volume after being placed inthe reactor. The quartz sand and Sibunit samples werepowders (0.25-0.50 mm size fraction).Propane and argon (99.9% pure) were deliveredfrom cylinders using a gas flow controller. Chlorine,obtained in an electrolyzer, was fed into the reactor asa chlorine-argon mixture. All pipelines and spacesintended for chlorine were protected against light. Theamount of chlorine obtained was determined with anaccuracy of ±2% from the electrochemical equivalentof chlorine and the amount of electricity that had beenpassed through the electrolyte (saturated sodium chloride solution). Propane and chlorine were fed at a rate of 200 and 50 (100) h-1, respectively; argon, at a rateof 400 h-1. The molar ratio of the gases in the reactionmixture was 38 : Cl2 : Ar = 2 : 0.5 (1) : 4. The totalGHSV of the gas mixture fed to the catalyst bed was650-700 h-1. The gaseous products were sampled atthe reactor outlet. The reaction products were analyzed on a Tsvet570 chromatograph (Tsvet, Russiausing a specialpurpose capillary column (20 m ×0.32 mm) with DVBPLOT as the stationary phaseand a flameionization detector.
References:
Testova;Shalygin;Maksimov;Paukshtis;Parmon [Kinetics and Catalysis,2015,vol. 56,# 4,p. 428 - 433][Kinet. Katal.,2015,vol. 56,# 4,p. 430 - 435,6]
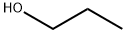
71-23-8
792 suppliers
$12.00/100g
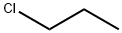
540-54-5
223 suppliers
$18.00/25mL

111-43-3
110 suppliers
$60.00/10g
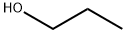
71-23-8
792 suppliers
$12.00/100g
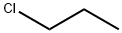
540-54-5
223 suppliers
$18.00/25mL

74-98-6
125 suppliers
$20.00/1ea
540-54-5
223 suppliers
$18.00/25mL

74-98-6
125 suppliers
$20.00/1ea
540-54-5
223 suppliers
$18.00/25mL

75-29-6
329 suppliers
$19.00/25mL