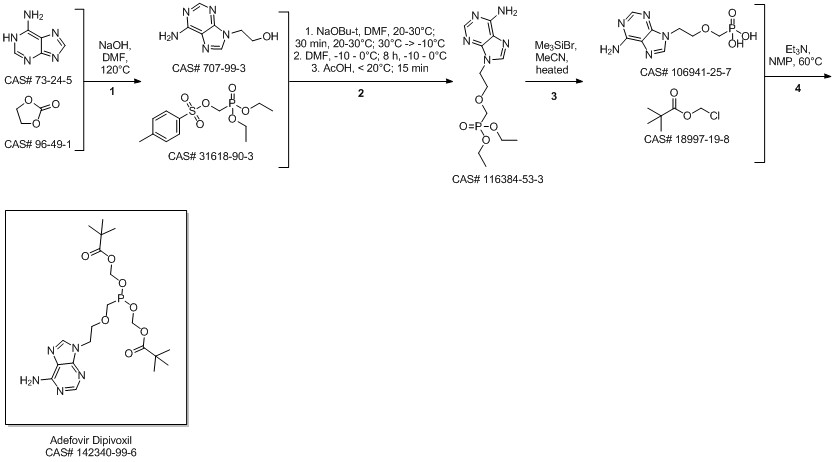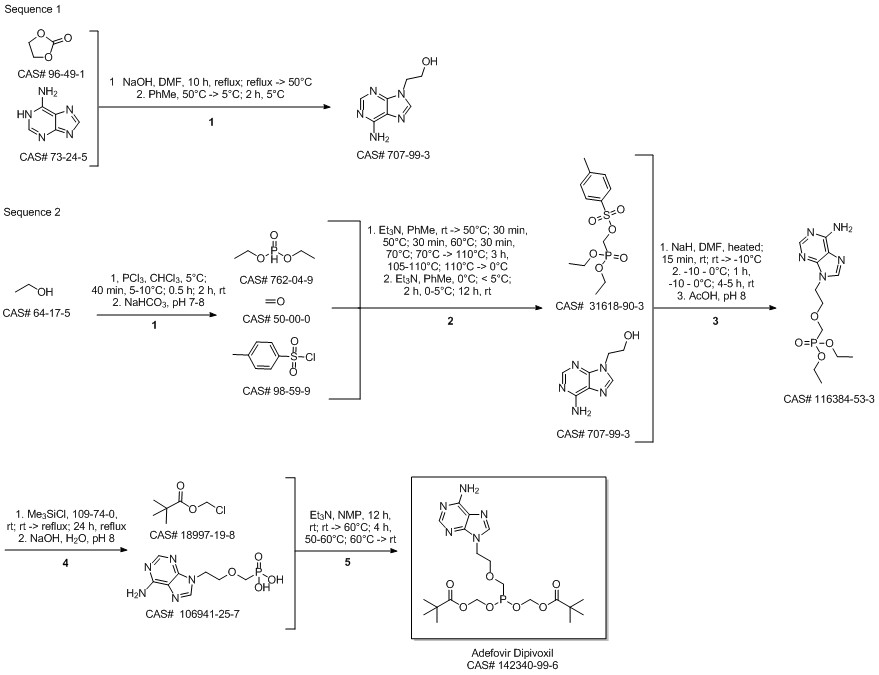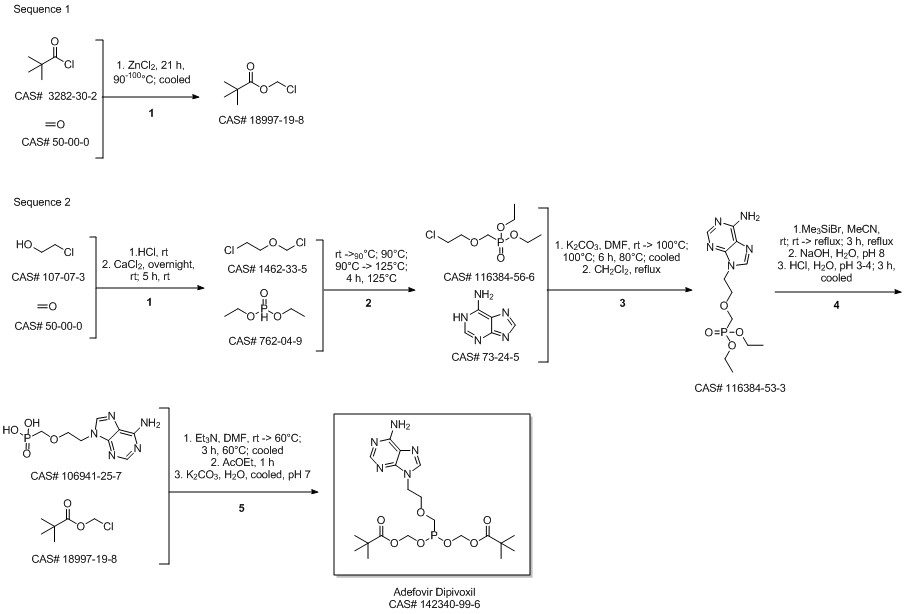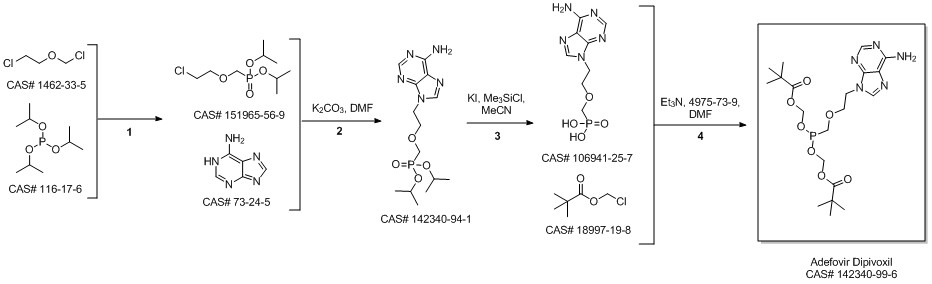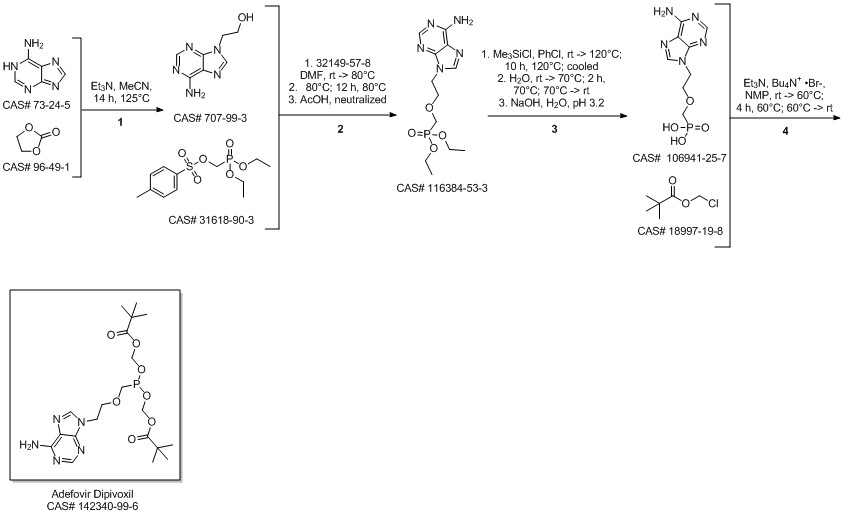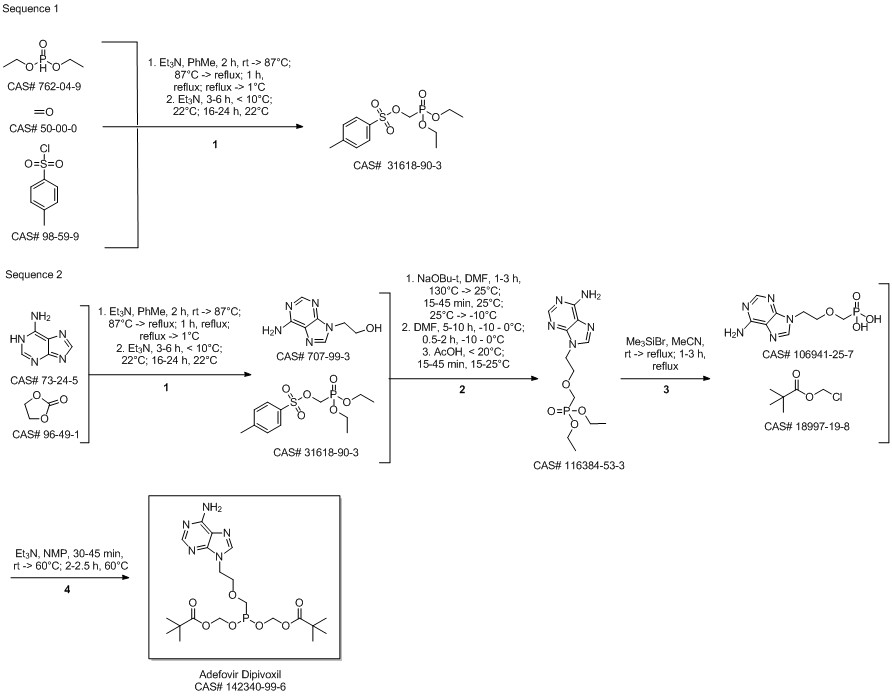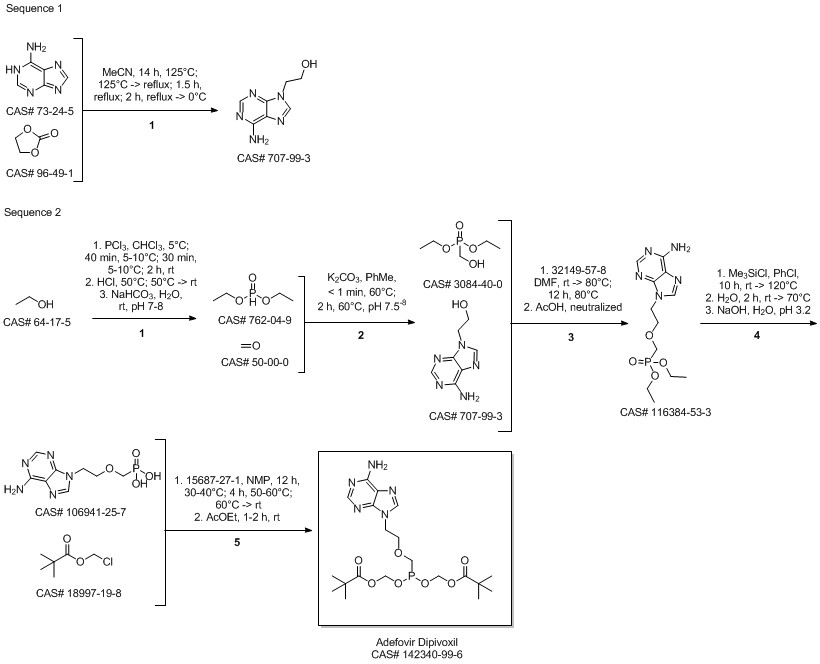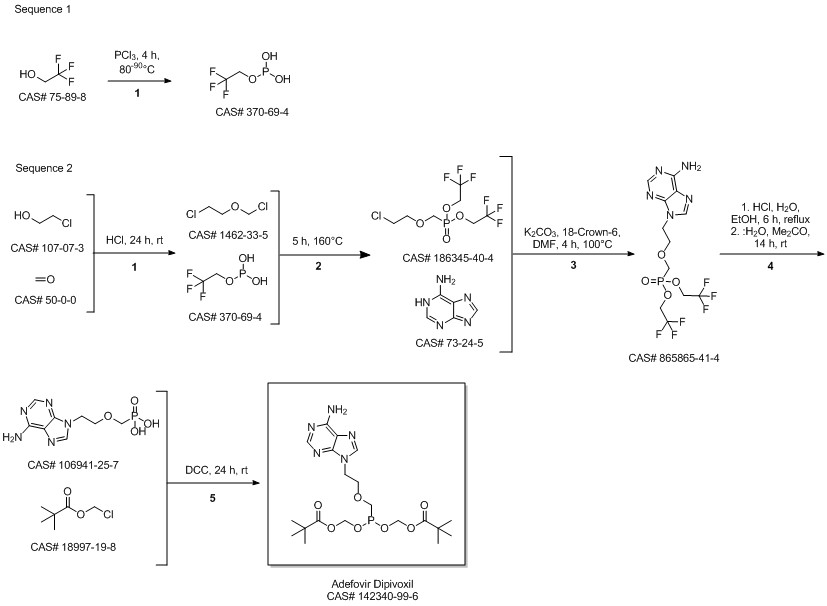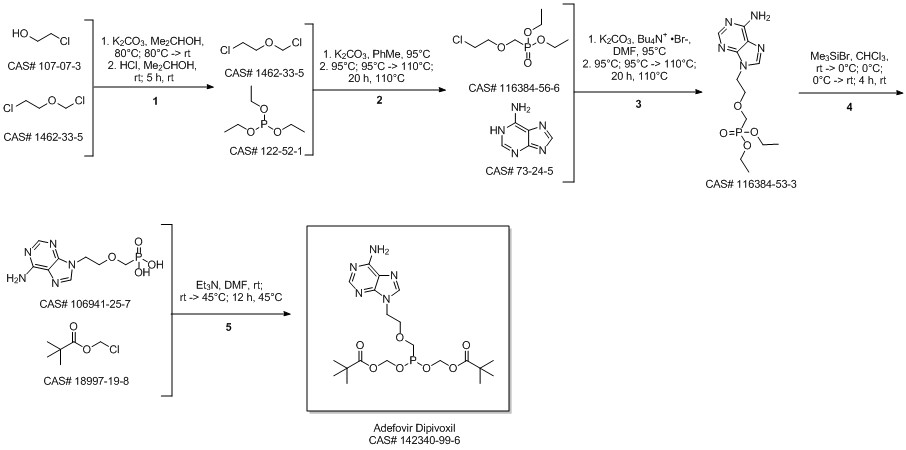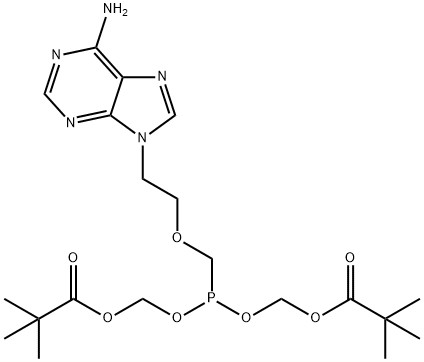
Adefovir dipivoxil synthesis
- Product Name:Adefovir dipivoxil
- CAS Number:142340-99-6
- Molecular formula:C20H32N5O8P
- Molecular Weight:501.47
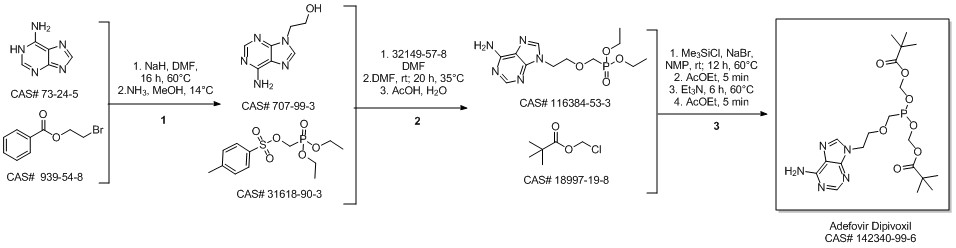
Roux, Loic; Priet, Stephane; Payrot, Nadine; Weck, Clement; Fournier, Maelenn; Zoulim, Fabien; Balzarini, Jan; Canard, Bruno; Alvarez, Karine. Ester prodrugs of acyclic nucleoside thiophosphonates compared to phosphonates: Synthesis, antiviral activity and decomposition study. European Journal of Medicinal Chemistry. Volume 63. Pages 869-881. 2013.
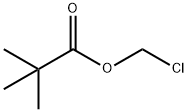
18997-19-8
317 suppliers
$5.00/5g
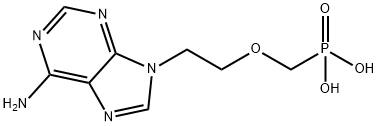
106941-25-7
338 suppliers
$5.00/250mg

142340-99-6
462 suppliers
$5.00/10mg
Yield:142340-99-6 78.1%
Reaction Conditions:
with triethylamine in 1-methyl-pyrrolidin-2-one at 50 - 55;Inert atmosphere;Large scale;Solvent;Reagent/catalyst;Temperature;
Steps:
1; 2; 3; 1
Adding N-methylpyrrolidone to a 50 L reactor and adding 1.70 kg of compound 2 with stirring.Triethylamine 2.52kg (4eq),Compound 3 5.62kg (6eq), protected with nitrogen, heated to 50 ~ 55 ° C,Incubate for 5-7 hours, HPLC monitor compound 4>74%; reaction solution is cooled to 25±5°C;The reaction solution was transferred to a 100 L swing drum and 34 kg (20 w/w) of ethyl acetate was added.Stir at 25 ± 5 ° C for 0.5 h; press filter, filter cake washed with 8.50 kg of ethyl acetate (5 w / w),The organic phase was combined to a 200 L kettle; the organic phase was added with 17.00 kg of purified water (10 w/w).Stir for 0.5 h, separate the liquid; transfer the aqueous phase to a 200 L kettle, add 17.00 kg of ethyl acetate (10 w/w), stir for 0.5 h, and separate the liquid; add the organic phase, add 17.00 kg of purified water (10 w / w), stir 0.5h, liquid separation; add 17.00kg purified water (10w / w) to the organic phase, stir for 0.5h, liquid separation; organic phase added 17.00kg saline (10w / w), stirred for 0.5h, liquid separation; organic addition 17.00kg saline solution (10w / w), stirred for 0.5h, liquid separation; organic phase added 17.00kg brine (10w / w), stirred for 0.5h, liquid separation; organic phase added 3.40kg (2w / w) anhydrous sulfuric acid Sodium, 170g (0.1w / w) activated carbon, stirred at 25 ± 5 ° C for 1h; filter press, filter cake washed with 3.40kg (2w / w) ethyl acetate; filtrate transferred to 200L kettle, 40 ± 5 ° C decompression Concentrate to about 8.5L (5v / w); add 15.30kg of methyl tert-butyl ether (9w / w), warmed to 43 ± 3 ° C, stirred for 0.5h; cooled to 5 ± 5 ° C, crystallization for 12h; centrifugation, The filter cake was washed with 1.70 kg of methyl tert-butyl ether (1 w/w); the filter cake was dried under vacuum at 40 ± 5 ° C to give compound 4 white 1.66 kg, yield 53.2%.Add 2.50 kg (2w/w) of acetone to a 10L four-necked flask, add 1.25kg of compound 4 with stirring, and raise the temperature to 40-45 °C; slowly add 2.50kg (2w/w) of methyl tert-butyl ether and heat up to 40 ~45 ° C, heat stirring for 0.5h; heat filtration to 20L glass crystallization kettle, heating to 40 ~ 45 ° C, heat preservation 0.5h; slow cooling; 38 ± 1 ° C added 0.3% seed crystal; slow cooling to 30 ~ 35 °C, keep warm crystal for 6h; slowly cool down to 5±5°C, heat crystallization for 12h; centrifugation, filter cake washed with 1.25kg (1w/w) of methyl tert-butyl ether; filter cake dried under vacuum at 40±5°C The compound 5 white crystalline powder was 1.00 kg, and the yield was 78.1%.
References:
Beijing Ji Mei Tang Pharmaceutical Co., Ltd.;Li Pei;He Jie;Jiang Aibin;Wang Hui CN110143983, 2019, A Location in patent:Paragraph 0020; 0051; 0054; 0055; 0057; 0060; 0061

18997-19-8
317 suppliers
$5.00/5g
![[[2-(6-Amino-9H-purin-9-yl)ethoxy]methyl]phosphonic acid diethyl ester](/CAS/GIF/116384-53-3.gif)
116384-53-3
172 suppliers
$27.00/1g

142340-99-6
462 suppliers
$5.00/10mg

18997-19-8
317 suppliers
$5.00/5g

106941-25-7
338 suppliers
$5.00/250mg
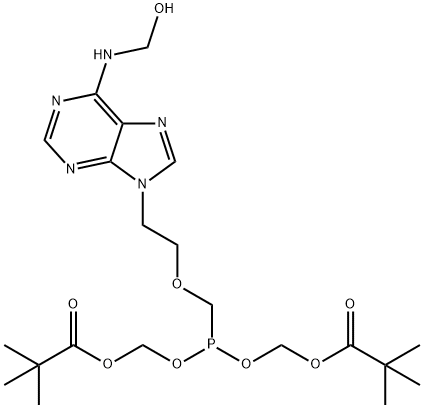
323201-04-3
24 suppliers
inquiry

142340-99-6
462 suppliers
$5.00/10mg
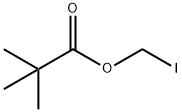
53064-79-2
197 suppliers
$19.00/1g

142340-99-6
462 suppliers
$5.00/10mg
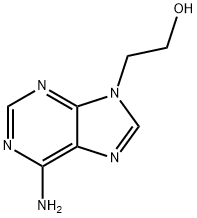
707-99-3
136 suppliers
$15.00/1g

142340-99-6
462 suppliers
$5.00/10mg