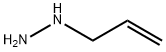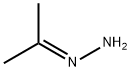
acetone hydrazone synthesis
- Product Name:acetone hydrazone
- CAS Number:5281-20-9
- Molecular formula:C3H8N2
- Molecular Weight:72.11
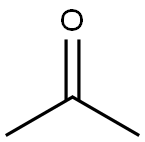
67-64-1
6 suppliers
$17.30/10ml

5281-20-9
19 suppliers
inquiry
Yield: 89%
Reaction Conditions:
with barium(II) oxide;hydrazine in methanol at -5 - 20;
Steps:
1.1 1.1 Preparation of isopropylidene-hydrazine
1.1 Preparation of isopropylidene-hydrazine A 500 ML 3-neck flask was fitted with a mechanical stirrer, thermometer, and addition funnel.The vessel was charged with 140 ML of methanol, and then cooled to -5° C. in an ice/salt bath. BaO (7.5 g, 50 mmole) was then added portion-wise over 5 minutes.Gas evolution and an exotherm were observed.A maximum temperature of 5° C. was reached.The reaction was cooled down to 0° C., and hydrazine monohydrate (32 g, 0.64 moles) was added in one portion, causing the mixture to warm to 5° C. The vessel was cooled down to 0° C. and the mixture was stirred for 10 minutes.A solution of acetone (37 g, 0.64 moles) in 40 ML of methanol was added drop-wise over 1 hour at 5° C. Stirring was continued, allowing the reaction to warm slowly to room temperature.Stirring was continued overnight, but if the reaction was allowed to proceed for only one hour, quite satisfactory yields were obtained. 1H NMR indicated the absence of acetone and a complete reaction.ether (300 ML) was added, which caused more solid to precipitate.Celite 545 and MgSO4 were added, the mixture was filtered through a bed of Celite on S&S sharkskin paper in a sintered glass funnel, and most of the ether was removed at or below room temperature on a rotary evaporator.The solution was then gently distilled.The distillate was placed in a clean flask and the remaining methanol was removed by rotovap evaporation without application of heat.The process was monitored by 1H NMR until <2.5% methanol remained. isopropylidene-hydrazine was obtained as a slightly cloudy colorless liquid (41 g, 89%), and used as such in subsequent reactions. 1H NMR (300 MHz, CDCl3)δ(ppm): 4.85 (br, 2H), 1.93 (s, 3H), 1.77 (s, 3H).
References:
Hormann, Robert Eugene;Chortyk, Orestes;Le, Dat Phat US2004/171651, 2004, A1 Location in patent:Page/Page column 27
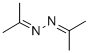
627-70-3
22 suppliers
$95.00/1g

5281-20-9
19 suppliers
inquiry
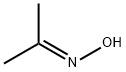
127-06-0
411 suppliers
$5.67/25g

5281-20-9
19 suppliers
inquiry
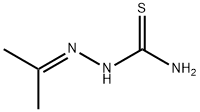
1752-30-3
202 suppliers
$30.00/20mg

5281-20-9
19 suppliers
inquiry