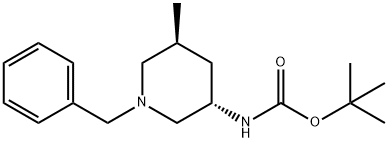
TERT-BUTYL (3S,5S)-1-BENZYL-5-METHYLPIPERIDIN-3-YLCARBAMATE synthesis
- Product Name:TERT-BUTYL (3S,5S)-1-BENZYL-5-METHYLPIPERIDIN-3-YLCARBAMATE
- CAS Number:951163-63-6
- Molecular formula:C18H28N2O2
- Molecular Weight:304.43
Yield:-
Reaction Conditions:
in 1,2-dimethoxyethane at 55 - 65;
Steps:
1.A
(3S,5S)-(1-Benzyl-5-methyl-piperidin-3-yl)-carbamic acid tert-butyl ester (7). A 50 L reactor is charged with 9.1 Kg of neat benzylamine. The reactor is brought to 55° C. and a solution of intermediate (6) (8.2 Kg) in 1,2-dimethoxyethane (DME) (14.1 Kg) is added to the reactor while maintaining a temperature of 60° C.+/-5° C. After complete addition of this solution, the reaction is stirred at 60° C.+/-5° C. for several hours and monitored for completion by TLC or HPLC. The reaction is cooled to ambient temperature and volatiles (DME) are removed by rotary evaporation under vacuum. The residue is diluted with 11.7 Kg of 15% (v/v) ethyl acetate/hexanes solution and treated, while agitating, with 18.7 Kg of 20% (wt) aqueous potassium carbonate solution. A triphasic mixture is obtained upon settling. The bottom aqueous phase is removed and the middle phase is set aside. The upper organic phase is collected and held for combination with extracts from additional extractions. The isolated middle phase is extracted twice again with 11.7 Kg portions of 15% (v/v) ethyl acetate/hexanes solution, each time combining the extracts with original organic phase. The combined organic extracts are transferred into a rotary evaporator and solvent is removed under vacuum until an oily residue remains. The residue is then purified via large-scale preparative chromatography to afford purified intermediate (7) as an oil.
References:
US2007/232650,2007,A1 Location in patent:Page/Page column 6
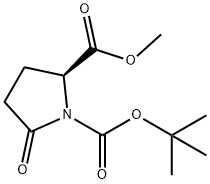
108963-96-8
287 suppliers
$5.00/1g
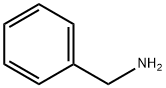
100-46-9
483 suppliers
$5.00/5G

74-88-4
347 suppliers
$15.00/10g

951163-63-6
12 suppliers
inquiry

24424-99-5
833 suppliers
$13.50/25G

951163-63-6
12 suppliers
inquiry
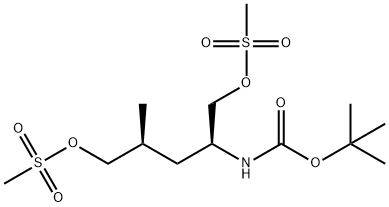
![1,1-Dimethylethyl N-[(1S,3S)-1-formyl-3-methyl-4-oxobutyl]carbamate](/CAS/20210305/GIF/1311194-65-6.gif)