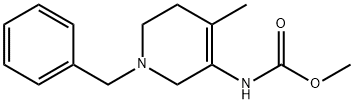
(1-Benzyl-4-Methyl-1,2,5,6-tetrahydropyridin-3-yl)carbaMic acid Methyl ester synthesis
- Product Name:(1-Benzyl-4-Methyl-1,2,5,6-tetrahydropyridin-3-yl)carbaMic acid Methyl ester
- CAS Number:923036-28-6
- Molecular formula:C15H20N2O2
- Molecular Weight:260.33
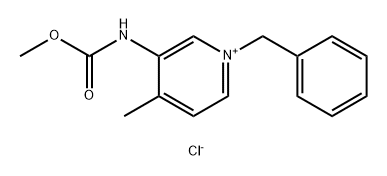
923036-29-7
1 suppliers
inquiry

923036-28-6
13 suppliers
inquiry
Yield:923036-28-6 72%
Reaction Conditions:
Stage #1: 1-benzyl-3-methoxycarbonylamino-4-methylpyridinium chloridewith sodium tetrahydroborate in ethanol at 20; for 16 h;
Stage #2: with water in ethanol;
Steps:
2
Example 2; Preparation of 1-benzyl-4-methyl-1 ,2,5,6-tetrahydro-pyridin-3-yl)-carbamic acid methyl ester; Benzyl chloride (2.1 mL, 18.3 mmol) was added to a slurry of (4-methyl-pyridin-3-yl)-carbamic acid methyl ester (3.16 g, 19.0 mmol) in toluene (15 mL) at 80 °C. A homogenous reaction mixture resulted after 10 minutes and then solids precipitated after 30 minutes. The slurry was stirred at 80 °C for 16 hours then cooled to room temp. The solids were filtered and washed with toluene. After drying, the product was isolated as a gray solid (4.17 g, 78 %).To a reaction mixture of 1-benzyl-3-methoxycarbonylamino-4-methyl-pyridinium chloride (4.0 g,13.0 mmol) in EtOH (ethanol) (20 mL) was added sodium borohydride (0.645 g, 17.0 mmol). Gas evolution was observed and the reaction was exothermic. The reaction mixture was stirred at room temp, for 16 hours. The reaction was quenched with H2O (10 mL), filtered through Celite and the Celite pad was washed with EtOH (2X10 mL). The solvent volume was reduced in vacuo. The resulting mixture was basified to pH 9 with 1 N sodium hydroxide then extracted with MTBE (methyl t butyl ether)(2X15 mL). The combined organic layers were washed with H2O (10 mL), dried (sodium sulfate), and concentrated to a yellow oil (2.44 g, 72 %).
References:
WO2007/12953,2007,A2 Location in patent:Page/Page column 19
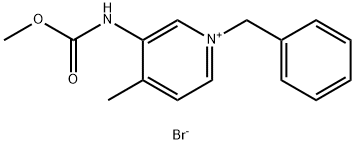
923036-27-5
5 suppliers
inquiry

923036-28-6
13 suppliers
inquiry