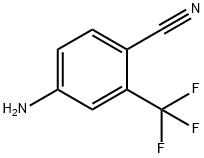
N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide synthesis
- Product Name:N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide
- CAS Number:90356-78-8
- Molecular formula:C18H14F4N2O2S
- Molecular Weight:398.37
Yield:90356-78-8 98%
Reaction Conditions:
Stage #1: 4-Fluorothiophenol;N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyloxirane-2-carboxamidewith methanol;sodium hydroxide;water at 0 - 25; for 3.5 h;
Stage #2: N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyloxirane-2-carboxamidewith hydrogenchloride;water at 5; pH=< 7; for 3 h;
Steps:
1 Preparation of N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropanamide
Example 1 Preparation of N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropanamide A solution of 4-fluorobenzenethiol (21g) in methanol (65 ml) was cooled to 0° C. and aqueous 50% sodium hydroxide (14 g) was added portionwise. The mixture was stirred at 0° C. for 30 minutes, then at 25° C. for 1 hour. To the mixture, N-[4-cyano-3-trifluoromethylphenyl]-2-methyloxiranecaboxamide (40 g) was added and the resulting mixture was stirred at room temperature for 2h. The reaction was determined to complete by TLC. Water (100 ml) was added to the mixture, followed by concentrated hydrochloric acid to a pH below 7. The solution was distilled under vacuum until no methanol distilled ceased, and the resulting suspension was stirred at 5° C. for 3 hours. The solid was collected by filtration and rinsed with water (2*40 ml). The solid was dried under vacuum at 50-60° C. to give 58 g (98%) of N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropanamide.
References:
US2007/27211,2007,A1 Location in patent:Page/Page column 2; 4
![3-[(4-Fluorophenyl)thio]-2-hydroxy-2-methylpropanoic acid](/CAS/GIF/339530-91-5.gif)
339530-91-5
42 suppliers
$227.00/250mg
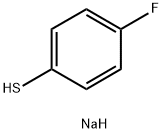
654-70-6
471 suppliers
$5.00/1g
![N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide](/CAS/GIF/90356-78-8.gif)
90356-78-8
218 suppliers
inquiry
![N-[4-Cyano-3-(trifluoromethyl)phenyl]-2-methacrylamide](/CAS/GIF/90357-53-2.gif)
90357-53-2
153 suppliers
$60.00/5G
![N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide](/CAS/GIF/90356-78-8.gif)
90356-78-8
218 suppliers
inquiry
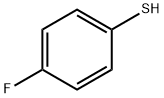
![N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide](/CAS/GIF/90357-51-0.gif)