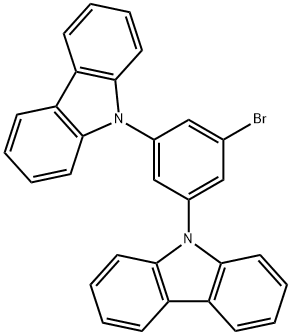
9,9'-(5-bromo-1,3-phenylene)bis(9H-carbazole) synthesis
- Product Name:9,9'-(5-bromo-1,3-phenylene)bis(9H-carbazole)
- CAS Number:750573-24-1
- Molecular formula:C30H19BrN2
- Molecular Weight:487.39
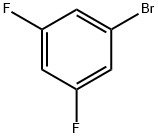
461-96-1
476 suppliers
$6.00/10g

86-74-8
336 suppliers
$10.00/5g

750573-24-1
89 suppliers
$45.00/10mg
Yield:750573-24-1 93%
Reaction Conditions:
Stage #1: 9H-carbazolewith potassium tert-butylate at 120 - 140;
Stage #2: 3,5-difluorobromobenzene for 2 h;
Steps:
Synthesis of Intermediate (G)
10.29 g (61.55 mmol) of carbazole and 7.25 g (64.63 mmol) of potassium tert-butoxide were mixed with 30 mL of 1,3-dimethyl-2-imidazolidinone at a temperature of 120° C. Subsequently, the temperature was raised to 140° C. Next, 5.94 g (30.78 mmol) of 1-bromo-3,5-difluorobenzene was added thereto, followed by stirring for 2 hours. Once the reaction was complete, the resulting mixture was cooled to room temperature, followed by addition of a sodium chloride aqueous solution. The organic layer was extracted by using dichloromethane, and the organic extract was dried with magnesium sulfate. Subsequently, the dry organic extract was filtered and concentrated under reduced pressure. The resulting residue was separated and purified by silica gel column chromatography to obtain a desired compound, 14.10 g of Intermediate (G) (at a yield of 93%). LC-Mass (calculated value: 486.07 g/mol, measured value: M+1=487 g/mol)
References:
US2019/229276,2019,A1 Location in patent:Paragraph 0386-0388
626-39-1
464 suppliers
$5.00/5g

86-74-8
336 suppliers
$10.00/5g

750573-24-1
89 suppliers
$45.00/10mg
28165-52-8
135 suppliers
$8.00/250mg

86-74-8
336 suppliers
$10.00/5g

750573-24-1
89 suppliers
$45.00/10mg
1435-51-4
275 suppliers
$5.00/5g

86-74-8
336 suppliers
$10.00/5g

750573-24-1
89 suppliers
$45.00/10mg

626-39-1
464 suppliers
$5.00/5g

750573-24-1
89 suppliers
$45.00/10mg