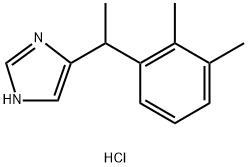
Medetomidine hydrochloride synthesis
- Product Name:Medetomidine hydrochloride
- CAS Number:86347-15-1
- Molecular formula:C13H17ClN2
- Molecular Weight:236.74
![5-[1-(2,3-DiMethylphenyl)ethenyl]iMidazole](/CAS/GIF/1021949-47-2.gif)
1021949-47-2
108 suppliers
$65.00/100mg

86347-15-1
242 suppliers
$5.00/10mg
Yield:86347-15-1 89%
Reaction Conditions:
Stage #1: 1-(4-imidazolyl)-1-(2,3-dimethylphenyl)ethylenewith hydrogen;5%-palladium/activated carbon in methanol;water at 44 - 46; under 1575.16 Torr; for 1.5 h;
Stage #2: with hydrogenchloride in water at -10 - 42; for 2.5 - 3 h;
Steps:
5.e
Example 5 (step e); Preparation of 5-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole hydrochloride hydrate 5-[1-(2,3-dimethylphenyl)vinyl]-1H-imidazole (80.0 g, 0.406 mol) and methanol (800 mL) were mixed in a 2-liter beaker. The reaction mixture was poured into a stirred laboratory autoclave. Palladium catalyst (1.9 g of 5% Pd/C) was weighed and immediately suspended in water (25 mL). The suspension was poured into the autoclave. The autoclave was closed and flushed 2 times with hydrogen to 0.21×106Pa. The hydrogen was supplied to autoclave to 0.21×106Pa. The reaction mixture was stirred and warmed to 44-46°C over 20-25 minutes. At the end of the hydrogenation, the hydrogen absorption ceased, and the pressure in the autoclave remained constant. The typical hydrogenation time was 1.5 hours. After hydrogenation the autoclave was flushed with nitrogen, the reaction mixture was filtered to remove the catalyst. The autoclave and the catalyst were washed with methanol (200 mL). The solvent was removed by distillation at a reduced pressure. Hydrochloric acid (4 M, 350 mL) was added to the distillation residue at 38 to 42°C. At first the reaction mixture was stirred without cooling for 30 minutes; then the reaction mixture was cooled to -5 to -10 °C for 2 to 2.5 hours. The precipitates were separated by filtration. The obtained intermediate 5-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole hydrochloride hydrate was dried at ambient temperature for 15 to 17 hours. The yield was 94.8g (89-98%) of an off-white powder.
References:
EP1918282,2008,A1 Location in patent:Page/Page column 6

1421259-21-3
1 suppliers
inquiry
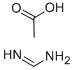
3473-63-0
493 suppliers
$5.00/25g

86347-15-1
242 suppliers
$5.00/10mg
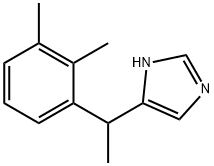
86347-14-0
184 suppliers
$5.00/10mg

86347-15-1
242 suppliers
$5.00/10mg
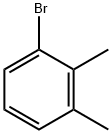
576-23-8
267 suppliers
$16.00/25g

86347-15-1
242 suppliers
$5.00/10mg
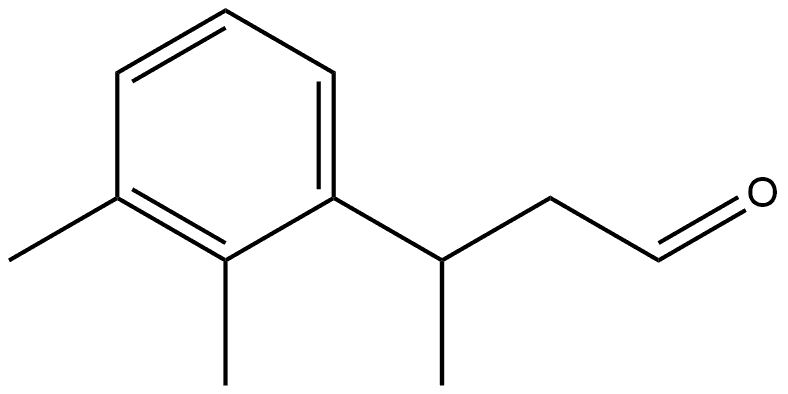
1421259-20-2
0 suppliers
inquiry

86347-15-1
242 suppliers
$5.00/10mg