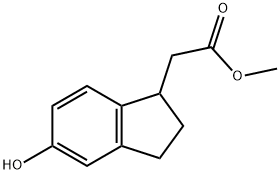
METHYL (5-HYDROXY-2,3-DIHYDRO-1H-INDEN-1-YL)ACETATE synthesis
- Product Name:METHYL (5-HYDROXY-2,3-DIHYDRO-1H-INDEN-1-YL)ACETATE
- CAS Number:856169-08-9
- Molecular formula:C12H14O3
- Molecular Weight:206.24
![Acetic acid, 2-[2,3-dihydro-5-(phenylmethoxy)-1H-inden-1-ylidene]-, methyl ester](/CAS/20210305/GIF/1187199-58-1.gif)
1187199-58-1
0 suppliers
inquiry

856169-08-9
9 suppliers
inquiry
Yield:856169-08-9 47%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in methanol; for 3 h;
Steps:
5; 31
(R)-Methyl 2-(5-hydroxy-2,3-dihydro-1H-inden-1-yl)acetate or (S)- methyl 2-(5-hydroxy-2,3-dihydro-1H-inden-1-yl)acetate (31.1 or 31.2). Under hydrogen, a mixture of methyl 2-(5-(benzyloxy)-2,3-dihydroinden-1-ylidene)acetate 5.3 (3.44 g, 11.7 mmol), Pd-C (1.38 g, 11.7 mmol) in MeOH (35 mL) was stirred for 3 hours. The resulting mixture was then filtered through silica gel to remove the Pd-C. After removing solvent, the residue was purified by chromatography (silica gel, 1 :2 EtOAc/hexane) to give racemic product in 47% yield. The racemate was separated by chiral chromatography (column: OD, 4% IPA/hexane) to give 31.1 (0.324 g, retention time: 23.9 minutes, >99% ee) and 31.2 (0.315 g, retention time: 30.4minutes, >99% ee). ; (R)-Methyl 2-(5-hydroxy-2,3-dihydro-1H-inden-1-yl)acetate or (S)- methyl 2-(5-hydroxy-2,3-dihydro-1H-inden-1-yl)acetate (5.4). Under hydrogen, a mixture of methyl 2-(5-(benzyloxy)-2,3-dihydroinden-1-ylidene)acetate 5.3 (3.44 g, 11.7 mmol), Pd-C (1.38 g, 11.7 mmol) in MeOH (35 mL) was stirred for 3 hours. The resulting mixture was then filtered through silica gel to remove the Pd-C. After removing solvent, the residue was purified by chromatography (silica gel, 1:2 EtOAc/hexane) to give a racemate in 47% yield. The racemate was separated by chiral chromatography (column: OD, 4% EPA/hexane) to give 5.4 (>99% ee).
References:
WO2009/111056,2009,A1 Location in patent:Page/Page column 273; 318
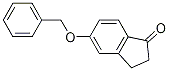
78326-88-2
13 suppliers
$299.00/1g

856169-08-9
9 suppliers
inquiry
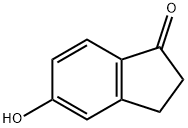
3470-49-3
165 suppliers
$10.00/1g

856169-08-9
9 suppliers
inquiry