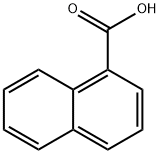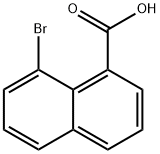
8-Bromo-1-naphthoic acid synthesis
- Product Name:8-Bromo-1-naphthoic acid
- CAS Number:1729-99-3
- Molecular formula:C11H7BrO2
- Molecular Weight:251.08
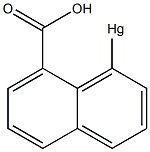
6314-27-8
4 suppliers
inquiry

1729-99-3
111 suppliers
$30.00/250mg
Yield:1729-99-3 84%
Reaction Conditions:
Stage #1:O-(hydroxymercuri)-1-naphtoic acid anhydride with acetic acid in water at 0; for 0.166667 h;
Stage #2: with bromine;sodium bromide in water at 0 - 100;
Steps:
5.1.31. 8-Bromo-1-naphthoic acid (27)
To a mixture of 3.9 mL (67 mmol) of acetic acid and 0.40 mL (22.2 mmol) of H2O was added with stirring 1.0 g (2.70 mmol) of anhydro-8-hydroxymercuri-1-naphthoic acid26 and the suspension was stirred at 0 °C for 10 min. A solution of 0.89 g (8.66 mmol) of NaBr in 3.2 mL of water and 0.43 g (2.70 mmol) of bromine were added sequentially and the reaction was slowly heated to 100 °C. After cooling to ambient temperature the mixture was poured into ice and 0.57 g (84%) of 8-bromo-1-naphthoic acid was filtered out as a cream colored solid: mp 173-174 °C (lit. mp 174-175 °C26); 1H NMR (500 MHz, DMSO-d6) δ 7.49 (t, J = 7.8 Hz, 1H), 7.61 (t, J = 7.3 Hz, 1H), 7.68 (d, J = 7.0 Hz, 1H), 7.96 (d, J = 7.3 Hz, 1H), 8.07 (d, J = 8.0 Hz, 1H), 8.11 (d, J = 8.0 Hz, 1H), 13.32 (s, 1H); 13C NMR (125.77 MHz, DMSO-d6) δ 119.3, 126.3, 127.6, 128.0, 128.3, 129.4, 131.2, 133.6, 133.9, 135.7, 171.3.
References:
Wiley, Jenny L.;Smith, Valerie J.;Chen, Jianhong;Martin, Billy R.;Huffman, John W. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 6,p. 2067 - 2081] Location in patent:experimental part
518-05-8
38 suppliers
inquiry
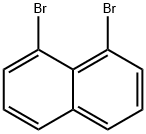
17135-74-9
273 suppliers
$8.00/250mg

1729-99-3
111 suppliers
$30.00/250mg
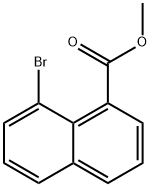
38058-95-6
23 suppliers
inquiry

1729-99-3
111 suppliers
$30.00/250mg
![Benz[cd]indol-2(1H)-one](/CAS/GIF/130-00-7.gif)