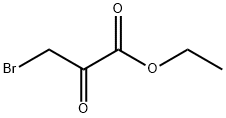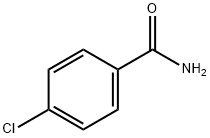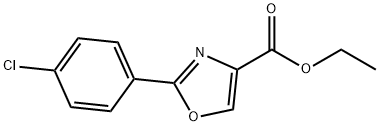
2-(4-CHLORO-PHENYL)-OXAZOLE-4-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:2-(4-CHLORO-PHENYL)-OXAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:78979-62-1
- Molecular formula:C12H10ClNO3
- Molecular Weight:251.67
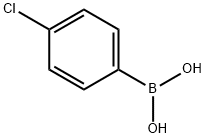
1679-18-1
606 suppliers
$10.00/1g
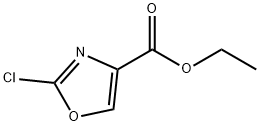
460081-18-9
166 suppliers
$10.00/250mg

78979-62-1
14 suppliers
inquiry
Yield:-
Reaction Conditions:
with 1,1'-bis-(diphenylphosphino)ferrocene;tetrakis(triphenylphosphine) palladium(0);potassium carbonate in water;toluene at 90; for 12 h;Suzuki Coupling;
Steps:
General procedure for the synthesis of intermediate 55 by Suzuki coupling (step c)
General procedure: Toan oven-dried round bottom flask charged withethyl 2-chlorothiazole-4-carboxylate(500 mg, 2.60 mmol, 1.0 equiv.), toluene (10 mL),Pd(PPh3)4(150 mg, 0.13 mmol, 0.05 equiv.), DPPF (43 mg, 0.08 mmol, 0.03 equiv.), and saturated aqueous K2CO3solution (720 mg, 5.20 mmol, 2.0 equiv. in 1mL H2O) was added 4-chlorophenylboronic acid(813 mg, 5.20 mmol, 2.0 equiv.).The reaction mixture was allowed to stir at 90oC for 12 h. The reaction mixture wascooled to room temperature, 2N NaOH solution and the resulting mixture was extracted with EtOAc. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified byMPLC (24g-silica-gel,n-hexanes/EtOAc)to affordethyl 2-(4-chlorophenyl)thiazole-4-carboxylate.
References:
Jung, Hui Jin;Nam, Eun Hye;Park, Jin Young;Ghosh, Prithwish;Kim, In Su [Bioorganic and Medicinal Chemistry Letters,2021,vol. 37,art. no. 127846] Location in patent:supporting information
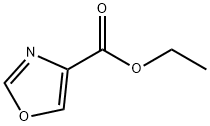
23012-14-8
210 suppliers
$5.00/250mg
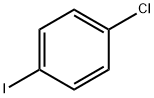
637-87-6
291 suppliers
$38.00/10g

78979-62-1
14 suppliers
inquiry

23012-14-8
210 suppliers
$5.00/250mg
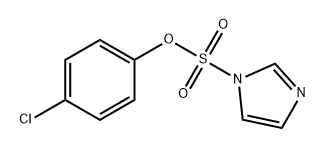
1198184-09-6
0 suppliers
inquiry

78979-62-1
14 suppliers
inquiry
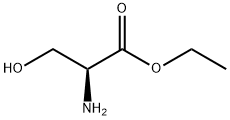
4117-31-1
17 suppliers
inquiry

78979-62-1
14 suppliers
inquiry