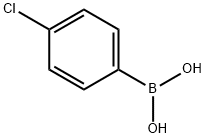
4-Chlorophenylboronic acid synthesis
- Product Name:4-Chlorophenylboronic acid
- CAS Number:1679-18-1
- Molecular formula:C6H6BClO2
- Molecular Weight:156.37
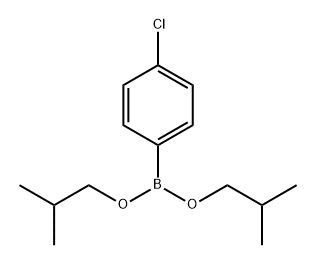
109409-95-2
0 suppliers
inquiry
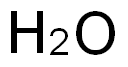
7732-18-5
485 suppliers
$13.50/100ML

1679-18-1
610 suppliers
$10.00/1g
Yield:1679-18-1 248 g
Reaction Conditions:
with hydrogenchloride at 20 - 30; pH=2 - 3; for 3 h;
Steps:
3.4; 3.5
4) Transfer the reaction mixture to a 3L reaction flask, slowly add 18% dilute hydrochloric acid to pH 2-3 with stirring, during which the temperature is controlled below 20 ° C, then keep warm at 20-30 ° C, and react for 3 hours; 5) Stop stirring, let the reaction mixture stand still, separate the upper organic phase and the lower aqueous phase, then extract the aqueous phase once with 200 g of toluene, separate the layers again, combine the organic phases, and combine the organic layers at not less than -0.08 The mixture was concentrated under reduced pressure under vacuum of MPa at a temperature of ≤ 65 ° C, and dried at 65-70 ° C to obtain 248 g of p-chlorophenylboronic acid in an off-white powder, yield 79%, purity ≥ 99%.
References:
CN108690063,2018,A Location in patent:Paragraph 0016
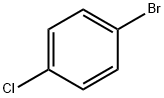
106-39-8
459 suppliers
$14.00/5g

1679-18-1
610 suppliers
$10.00/1g
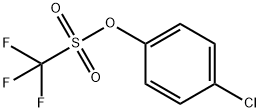
29540-84-9
36 suppliers
$48.00/5g

1679-18-1
610 suppliers
$10.00/1g
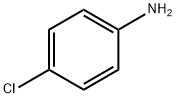
106-47-8
490 suppliers
$5.00/5G

1679-18-1
610 suppliers
$10.00/1g
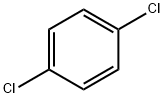
106-46-7
533 suppliers
$10.00/5g
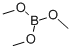
121-43-7
370 suppliers
$14.00/25mL

1679-18-1
610 suppliers
$10.00/1g