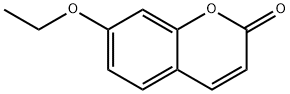
7-Ethoxycoumarin synthesis
- Product Name:7-Ethoxycoumarin
- CAS Number:31005-02-4
- Molecular formula:C11H10O3
- Molecular Weight:190.2
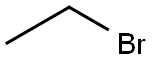
74-96-4
441 suppliers
$9.00/5g
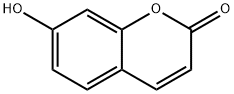
93-35-6
496 suppliers
$6.00/100mg

31005-02-4
157 suppliers
$15.00/100mg
Yield: 92%
Reaction Conditions:
Stage #1:7-hydroxy-2H-chromen-2-one with potassium carbonate in acetone for 0.25 h;Inert atmosphere;Reflux;
Stage #2:ethyl bromide in acetoneInert atmosphere;Reflux;
Steps:
General procedure for the synthesis of 7-O-alkylumbelliferone (1a-j):
General procedure: In a 50 mL two necked round-bottomed flask equipped with a magnetic stirrer, a condenser, and a nitrogen inlet,umbelliferone (1 equiv.), K2CO3 (3 equiv.) and 10 mL of anhydrous acetone were added. The mixture was heated underreflux for 15 min under nitrogen atmosphere and cooledto room temperature before the dropwise addition of alkylbromide (1.5 equiv.). The resulting mixture was heated underreflux for another 2-6 h. The reaction was quenched withwater (10 mL), followed by extraction with EtOAc (2×50 mL)and washed with brine solution. After drying (Na2SO4) andremoval of the solvent, the residue was purified by columnchromatography using hexane/EtOAc as mobile phase (gradientelution), to afford the corresponding compounds 1aj14.All the derivatives were characterized by 1H, 13C NMRand mass data.
References:
Yadav, Nisha;Auti, Prashant;George, Ginson;Paul, Atish T. [Journal of the Indian Chemical Society,2020,vol. 97,# 8,p. 1265 - 1271]

93-35-6
496 suppliers
$6.00/100mg
75-03-6
360 suppliers
$11.00/5g

31005-02-4
157 suppliers
$15.00/100mg

93-35-6
496 suppliers
$6.00/100mg
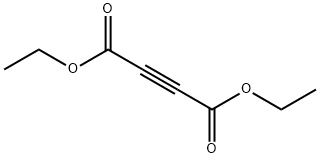
762-21-0
248 suppliers
$23.30/5g

31005-02-4
157 suppliers
$15.00/100mg

93-35-6
496 suppliers
$6.00/100mg

31005-02-4
157 suppliers
$15.00/100mg
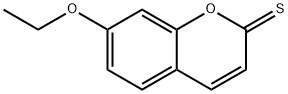
1261392-51-1
0 suppliers
inquiry

31005-02-4
157 suppliers
$15.00/100mg