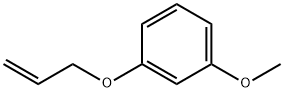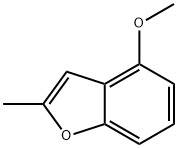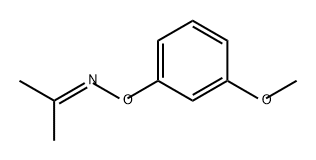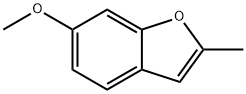
6-METHOXY-2-METHYLBENZOFURAN synthesis
- Product Name:6-METHOXY-2-METHYLBENZOFURAN
- CAS Number:29040-48-0
- Molecular formula:C10H10O2
- Molecular Weight:162.19
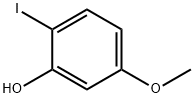
41046-70-2
48 suppliers
$27.00/100mg
74-99-7
99 suppliers
$45.00/1ml

29040-48-0
25 suppliers
inquiry
Yield:29040-48-0 74%
Reaction Conditions:
with copper(l) iodide;N,N,N',N'-tetramethylguanidine;bis-triphenylphosphine-palladium(II) chloride in DMF (N,N-dimethyl-formamide) at -78 - 20; for 18 h;
Steps:
12a Example 12(a): 6-methoxy-2-methylbenzofuran
A pressure tube was charged with a stir bar 2-lodo-5-methoxyphenol (500mg, 2. 0mmol) (prepared according to Heterocycles 45, (6), 1997,1137), Cl2Pd (PPh3) 2 (70mg, [0.] 1mmol), Cul (19mg, [0.] [1MMOL),] 1,1, 3, [3-TETRAMETHYLGUANIDINE] (2. 5ml, 20. 0mmol) and DMF (10 [ML),] then cooled [TO-78°] while propyne gas was condensed. The tube was sealed and allowed to warm to room temperature. After 18 hours, the contents of the tube were poured into sat NaCI solution and extracted with EtOAc (2x). The combined organic layers were washed with brine (3x), dried [(MGS04)] and concentrated under reduced pressure. The residue was flash chromatographed on silica gel eluting 3: 1 (hexanes/ethyl acetate) to give 239mg (74%) of an amber [LIQUID.'H] NMR [(DMSO-D6) No. ]7.31 [(1 H,] d, J = 8.3 Hz), 6.95 [(1 H,] [D,] J = 2.2 Hz), 6.80 (1 H, dd, J = 2.2, 8.3 Hz), 6.27 (1 H, s), 3.85 (3H, s), 2.40 (3H, s). AnaL Calcd for [C10H1002 ]0.05 hexanes: [C,] 74.30 ; H, 6.48. Found: [C,] [74. 63] ; H, 6.48.
References:
WO2003/106462,2003,A1 Location in patent:Page 50; 51
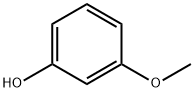
150-19-6
336 suppliers
$6.00/5g

29040-48-0
25 suppliers
inquiry
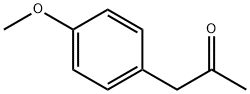
122-84-9
472 suppliers
$5.00/5g

29040-48-0
25 suppliers
inquiry