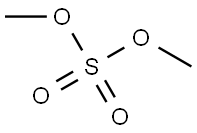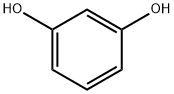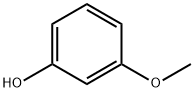
3-Methoxyphenol synthesis
- Product Name:3-Methoxyphenol
- CAS Number:150-19-6
- Molecular formula:C7H8O2
- Molecular Weight:124.14
Organicum. Practical Handbook of Organic Chemistry, by Heinz Becker, Werner Berger and Günter Domschke, Addison-Wesley Pub. Co, 206-207, (1973)
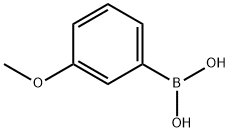
10365-98-7
417 suppliers
$9.00/10g

150-19-6
336 suppliers
$6.00/5g
Yield:150-19-6 99%
Reaction Conditions:
with dihydrogen peroxide in ethanol at 20; for 0.0166667 h;Green chemistry;Solvent;
Steps:
General procedure for the oxidation using H2O2
General procedure: A 25 ml flask was charged with phenylboronic acid (1 mmol). Then 1.6 mL H2O2 and 1 mL EtOH were added under stirring. The reaction was stirred for 1 min, then quenched by water (10 ml). The aqueous layer was extracted with 20 mL ethyl acetate for three times. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The pure product was obtained without flash column chromatography and the purity was determine by TLC.
References:
Dong, Zhenhua;Liu, Mengmeng;Pan, Hongguo [Arkivoc,2021,vol. 2021,# 8]
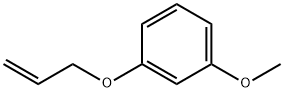
37592-19-1
0 suppliers
inquiry

150-19-6
336 suppliers
$6.00/5g
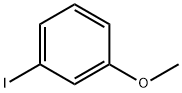
766-85-8
192 suppliers
$22.00/5g

150-19-6
336 suppliers
$6.00/5g
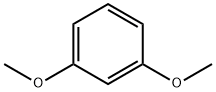
151-10-0
448 suppliers
$5.00/10g

150-19-6
336 suppliers
$6.00/5g