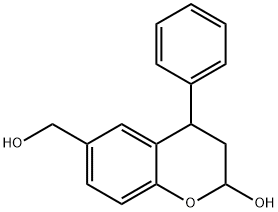
6-(hydroxyMethyl)-4-phenylchroMan-2-ol synthesis
- Product Name:6-(hydroxyMethyl)-4-phenylchroMan-2-ol
- CAS Number:959624-24-9
- Molecular formula:C16H16O3
- Molecular Weight:256.3

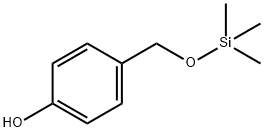
14371-10-9
319 suppliers
$6.00/25g
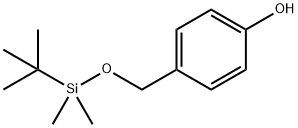
126070-20-0
27 suppliers
$140.00/100mg

959624-24-9
22 suppliers
inquiry
Yield:959624-24-9 66%
Reaction Conditions:
with 1-methyl-piperazine in toluene at 100 - 110; for 21 h;
Steps:
4 Example 4 Preparation of Feso Chromenyl from 4-t-butyl-dimethylsilyloxymethyl-phenol
20 g of 4-t-butyl-dimethylsilyloxymethyl-phenol, 240 ml of toluene and 20.7 g of N-methyl-piperazine are introduced into a 500 ml flask. The mixture is heated to 100° C. and 13.6 g of trans-cinnamaldehyde are dripped thereonto. It is maintained at 100° C. for 16 hours, then the temperature is brought to 110° C. and it is maintained in such conditions for another 5 hours. The reaction mixture is cooled to 40° C. and 100 ml of deionised water are added thereto, it is stirred for 30′ and the phases are left to separate. 60 ml of a 1 M solution of tetrabutylammonium fluoride under THF are added to the organic phase, the reaction mixture then being brought to 50° C. for two hours. Then it is cooled to 30° C. and 100 ml of deionised water are added, it is stirred for 30′ and the phases are left to separate. 200 ml of a 5% aqueous HCl solution and 60 ml of acetate ethyl are added to the organic phase. The mixture is brought to 50° C. and such conditions are maintained for one hour. Once the mixture is cooled to 25° C., the phases are left to separate and the organic phase is washed in sequence using 200 ml of an aqueous solution of sodium bicarbonate at 10% and twice with 200 ml of deionised water. The organic phase is thus concentrated under vacuum at 40° C. up to residue, which is recovered with 20 ml of toluene and 10 ml of ethyl acetate, reconcentrating once again up to residue. The obtained residue is crystallised by 30 ml of toluene and 13 ml of ethyl acetate. This enables obtaining 14 g of a yellow solid constituted by the expected product (66% yield).
References:
US2013/79532,2013,A1 Location in patent:Paragraph 0075
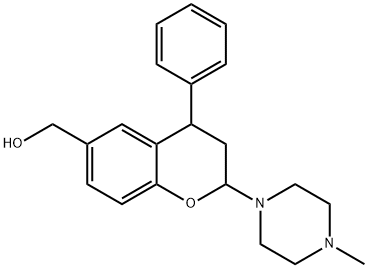
959624-43-2
7 suppliers
inquiry

959624-24-9
22 suppliers
inquiry

14371-10-9
319 suppliers
$6.00/25g
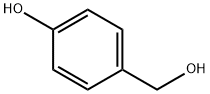
623-05-2
678 suppliers
$5.00/100mg

959624-24-9
22 suppliers
inquiry

623-05-2
678 suppliers
$5.00/100mg

959624-24-9
22 suppliers
inquiry