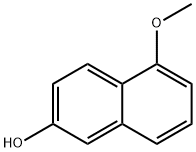
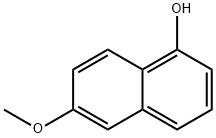
6-Hydroxy-2-methoxynaphthalene synthesis
- Product Name:6-Hydroxy-2-methoxynaphthalene
- CAS Number:150712-57-5
- Molecular formula:C11H10O2
- Molecular Weight:174.2
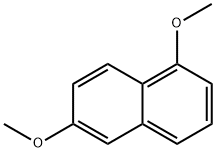
3900-49-0
205 suppliers
$10.00/1g

150712-57-5
7 suppliers
inquiry
Yield:150712-57-5 27%
Reaction Conditions:
with bromocatecholborane in 1,2-dichloro-ethane; for 72 h;Heating / reflux;
Steps:
3 EXAMPLE 3; N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-5-methoxy-2-naphthamide hydrochloride
Bromocatecholborane (4.11 g, 20.7 mmol) is added to a solution of 1,6-dimethoxynaphthalene (3.89 g, 20.7 mmol) in dichloroethane (60 mL). The resulting solution is heated to reflux for 72 hours, allowed to cool, and then poured into 1N HCl. The resulting mixture is extracted three times with CH2Cl2. The combined organic layers are dried (Na2SO4), filtered and concentrated to dryness. The product is purified by three columns (step gradient of 50-60-65%CH2Cl2 in hexanes) to give 5-methoxy-2-hydroxy naphthalene as an oil that solidifies on sitting (955 mg, 27%). 1H NMR (300 MHz, CDCl3) δ8.17, 7.2-7.4, 7.0-7.1, 6.66, 5.08, 3.97.
References:
US2003/236270,2003,A1 Location in patent:Page 38
32940-15-1
313 suppliers
$13.43/250mgs:

150712-57-5
7 suppliers
inquiry
3470-50-6
225 suppliers
$6.00/1g

150712-57-5
7 suppliers
inquiry
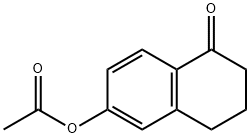
53473-35-1
5 suppliers
inquiry

150712-57-5
7 suppliers
inquiry
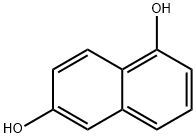
575-44-0
357 suppliers
$7.00/10g
22604-07-5
96 suppliers
$22.00/100mg

150712-57-5
7 suppliers
inquiry