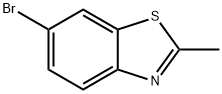
6-BROMO-2-METHYL-1,3-BENZOTHIAZOLE synthesis
- Product Name:6-BROMO-2-METHYL-1,3-BENZOTHIAZOLE
- CAS Number:5304-21-2
- Molecular formula:C8H6BrNS
- Molecular Weight:228.11
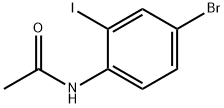
562080-91-5
34 suppliers
$251.68/250mg

5304-21-2
111 suppliers
$14.00/250mg
Yield:5304-21-2 29.5%
Reaction Conditions:
Stage #1:N-(4-bromo-2-iodophenyl)acetamide with copper(l) iodide;sodiumsulfide nonahydrate in N,N-dimethyl-formamide at 80; for 8 h;
Stage #2: with hydrogenchloride in water;N,N-dimethyl-formamide at 80; for 12 h;
Steps:
5 Synthesis of 6-bromo-2-methyl-benzothiazole (Compound 11)
Compound 10 (678 mg, 2.00 mmol)Was dissolved in dimethylformamide (4.00 mL)Sodium sulfide nonahydrate (721 mg, 6.00 mmol),Copper (I) iodide (38.0 mg, 0.200 mmol) was added,And the mixture was stirred at 80 ° C. for 8 hours.After returning to room temperature,Concentrated hydrochloric acid (1.6 mL) was added,And the mixture was further stirred at 80 ° C. for 12 hours.Saturated aqueous sodium hydrogen carbonate solution (20 mL) was added,And extracted with ethyl acetate (50 mL × 2).The organic layer was washed with saturated brine,After dehydration with anhydrous magnesium sulfate,The solvent was distilled off under reduced pressure,The residue was subjected to silica gel column chromatography using ethyl acetate / hexane (1/4 (volume ratio)) as an elution solvent,Compound 11 was obtained in a yield of 134 mg (29.5%).
References:
Kyoto University;Nihon Medi-Physics Co.,Ltd.;Saji, Hideo;Ono, Masahiro;Inohara, Tadashi;Seki, Ikuya JP2016/79108, 2016, A Location in patent:Paragraph 0055
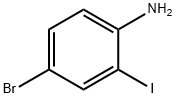
66416-72-6
189 suppliers
$6.00/250mg
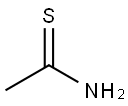
62-55-5
551 suppliers
$9.00/5 g

5304-21-2
111 suppliers
$14.00/250mg
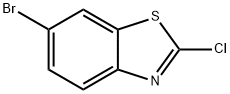
80945-86-4
200 suppliers
$8.00/1g
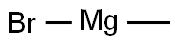
75-16-1
270 suppliers
$12.00/10ml

5304-21-2
111 suppliers
$14.00/250mg
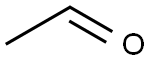
75-07-0
418 suppliers
$14.00/5mL
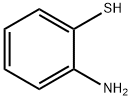
137-07-5
302 suppliers
$10.00/5g

5304-21-2
111 suppliers
$14.00/250mg
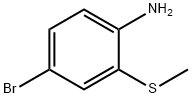
475089-07-7
22 suppliers
inquiry

5304-21-2
111 suppliers
$14.00/250mg