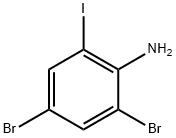
4-BROMO-2-IODOANILINE synthesis
- Product Name:4-BROMO-2-IODOANILINE
- CAS Number:66416-72-6
- Molecular formula:C6H5BrIN
- Molecular Weight:297.92
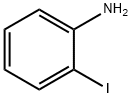
615-43-0
417 suppliers
$5.00/5g

66416-72-6
190 suppliers
$6.00/250mg
Yield:66416-72-6 92%
Reaction Conditions:
with ammonium molybdate;sodium perborate;acetic acid;potassium bromide at 0 - 20; for 3 h;
Steps:
44.a EXAMPLE 44; Step a) N- (5-BROMO-4'-FLUORO-1, 1'-BIPHENYL-2-YL) PROPANAMIDE
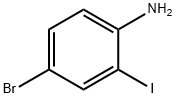
A vigorously stirred suspension of 2-iodoaniline (10. 0 g, 45.7 MMOL), potassium bromide (6.52 g, 54.8 MMOL), and ammonium molybdat tetrahydrate (0.57 g, 0.46 MMOL) in acetic acid (54 mL) was cooled to 0°C, treated with sodium perborate (7.74 g, 50.3 MMOL), and stirred for two hours as the reaction warmed to room temperature. After adding water and stirring one additional hour, the reaction mixture was poured into ice-water and filtered. The filtered solid was washed with water, dissolved in ethyl acetate (250 mL), and washed with a saturated, aqueous, potassium carbonate solution (2 x 75 mL). The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford the intermediate, 4 bromo-2- iodoaniline, as a red solid (12.56 g, 92%). [0196] 5-Bromo-4'-fluoro-1, 1'-biphenyl-2-ylamine was prepared from the above intermediate, 4-bromo-2-iodoaniline (12.6 g, 42.2 MMOL), 4-FLUOROPHENYLBORONIC acid (5.9 g, 42 MMOL), [1,1'-bis (diphenylphosphino) ferrocene] DICHLOROPALLADIUM (II) COMPLEX with DICHLOROMETHANE (0.69 g, 0.84 MMOL), and a 5 N aqueous sodium hydroxide solution (85 mL, 425 MMOL) according to the procedure and in the same manner as described in Example 32, Method A, Step a. [0197] The title compound was prepared from the above 5-bromo-4'-fluoro-1, 1'- biphenyl-2-ylamine (10.1 g, 37.8 MMOL), propionic anhydride (5.33 mL, 41.6 MMOL), 4- (N, N-dimethylamino) pyridine (0.46 g, 3.8 MMOL), and pyridine (12.3 mL, 151 MMOL), according to the same procedure, to yield N- (5-BROMO-4'-FLUORO-1, 1'-BIPHENYL-2- YL) propanamide as a solid (7.74 g, 64% from 4-bromo-2-iodoaniline), m. p. 133°C ; MS [(-ESI), M/Z] : 320 [M-H]- ; 'H NMR (500 MHz, DMSO-d6) S : 9.21 (s, 1 H), 7.52 (dd, J = 2.4, 8.5 Hz, 1 H), 7.47 (d, J = 2.2 Hz, 1H), 7.43-7. 38 (m, 3H), 7.28-7. 23 (m, 2H), 2.13 (q, J = 7.5 Hz, 2H), 0.95 (t, J = 7. 5 HZ, 3H); Anal. CALCD for C15H13BRFNO : C, 55.92 ; H, 4.07 ; N, 4.35. Found: C, 55.79 ; H, 4.08 ; N, 4.31
References:
WO2004/50631,2004,A1 Location in patent:Page 116
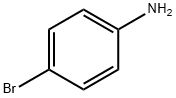
106-40-1
468 suppliers
$12.00/5g

66416-72-6
190 suppliers
$6.00/250mg

106-40-1
468 suppliers
$12.00/5g

66416-72-6
190 suppliers
$6.00/250mg
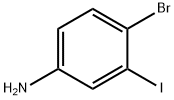
63037-64-9
40 suppliers
inquiry

615-43-0
417 suppliers
$5.00/5g

66416-72-6
190 suppliers
$6.00/250mg
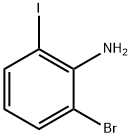
84483-27-2
53 suppliers
$38.00/100mg