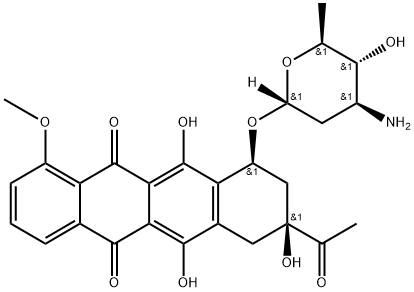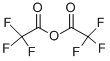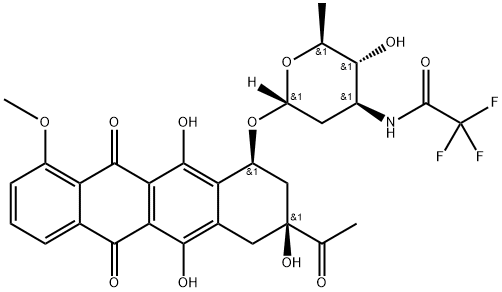
(8S-cis)-8-acetyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-[(trifluoroacetyl)amino]-alpha-L-arabino-hexopyranosyl]oxy]naphthacene-5,12-dione synthesis
- Product Name:(8S-cis)-8-acetyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-[(trifluoroacetyl)amino]-alpha-L-arabino-hexopyranosyl]oxy]naphthacene-5,12-dione
- CAS Number:57918-22-6
- Molecular formula:C29H28F3NO11
- Molecular Weight:623.53
Yield:57918-22-6 100%
Reaction Conditions:
Stage #1: 4’-epidaunorubicin;trifluoroacetic anhydride in ethyl acetate;Inert atmosphere;
Stage #2: with sodium hydroxide in methanol;ethyl acetate at 20; for 1 h;
Steps:
1 (1) Synthesis of Triflloxacin Tablets:
1.007 g (1.77 mmol) of epirubicin II was suspended in ethyl acetate (20 mL), and 1 mL of trifluoroacetic anhydride was added under nitrogen, and the solution became clear. The solution was washed with saturated sodium bicarbonate solution until the solution was weakly alkaline. The ethyl acetate layer was separated and a solution of NaOH (106 mg, 2.65 mmol) in methanol (5 mL) was added to the solution and stirred at room temperature for 1 hour. TLC Substrate disappears. The reaction solution was washed twice with a saturated aqueous sodium chloride solution, and the organic layer was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 1.1 g of trifluoroacetylamycin daunorubicin (III) in a yield of 100%. Melting point: 154-155 ° C.
References:
CN104098627,2017,B Location in patent:Paragraph 0030; 0031; 0032
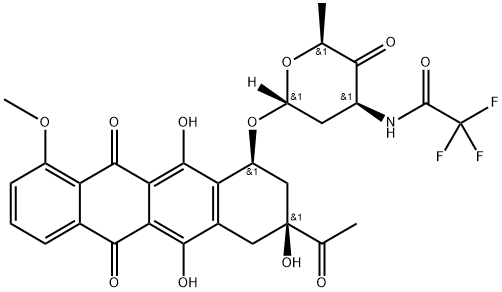
79441-78-4
7 suppliers
inquiry
![(8S-cis)-8-acetyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-[(trifluoroacetyl)amino]-alpha-L-arabino-hexopyranosyl]oxy]naphthacene-5,12-dione](/CAS/20211123/GIF/57918-22-6.gif)
57918-22-6
6 suppliers
inquiry
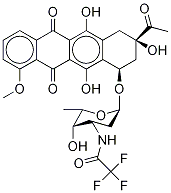
26388-52-3
14 suppliers
$90.00/1mg
![(8S-cis)-8-acetyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-[(trifluoroacetyl)amino]-alpha-L-arabino-hexopyranosyl]oxy]naphthacene-5,12-dione](/CAS/20211123/GIF/57918-22-6.gif)
57918-22-6
6 suppliers
inquiry
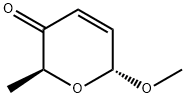
55102-46-0
0 suppliers
inquiry
![(8S-cis)-8-acetyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-[(trifluoroacetyl)amino]-alpha-L-arabino-hexopyranosyl]oxy]naphthacene-5,12-dione](/CAS/20211123/GIF/57918-22-6.gif)
57918-22-6
6 suppliers
inquiry
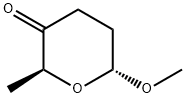
59492-30-7
0 suppliers
inquiry
![(8S-cis)-8-acetyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-[(trifluoroacetyl)amino]-alpha-L-arabino-hexopyranosyl]oxy]naphthacene-5,12-dione](/CAS/20211123/GIF/57918-22-6.gif)
57918-22-6
6 suppliers
inquiry