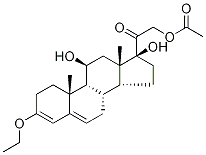
(11β)-17-Dihydroxy-pregna-3,5-dien-20-one 21-(Acetyloxy)-3-ethoxy-11 synthesis
- Product Name:(11β)-17-Dihydroxy-pregna-3,5-dien-20-one 21-(Acetyloxy)-3-ethoxy-11
- CAS Number:56736-68-6
- Molecular formula:C25H36O6
- Molecular Weight:432.55
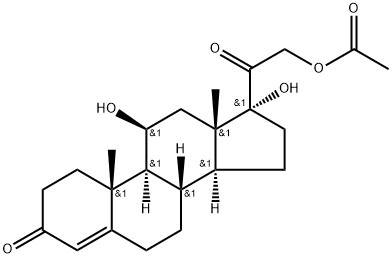
50-03-3
599 suppliers
$32.70/1g
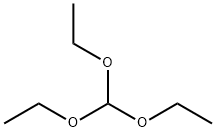
122-51-0
519 suppliers
$10.00/5ml

56736-68-6
10 suppliers
$500.20/5MG
Yield:56736-68-6 66%
Reaction Conditions:
with toluene-4-sulfonic acid in tetrahydrofuran;ethanol at 20; for 4 h;
Steps:
2
To a solution of 001-2 (17 g, 42 mmol) in EtOH (19 mL) and THF (190 mL) was added CH(OEt)3 (38.6 mL, 231 mmol) and p-TsOH (463 mg, 2.31 mmol). The mixture was stirred at 20 °C for 4 h. TLC (petroleum ether: ethyl acetate= 1:1) showed that the starting material was consumed completely. Then the mixture was diluted with EtOAc (200 mL), and washed with aqueous NaHCO3 solution. The organic phase was dried over Na2SO4 and evaporated to give the crude product, which was purified by column chromatography on silica gel (eluent: petroleum ether: ethyl acetate=7:1) to afford 001-3 (12 g, 66.0%) as white solid. ‘H NMR (001-3): (300 MHz, DMSO) ? 5.39 (s, 1H), 5.13-5.07 (m, 2H), 5.07-5.0 1 (m, 1H), 4.77-4.7 1 (d, J=23.4Hz, 1H), 4.27 (m, 1H), 4.18-4.17 (m, 1H), 3.73-3.67 (m, 2H), 2.50-2.19 (m, 2H), 2.09 (s, 3H), 2.03-1.92 (m, 4H), 1.80-1.60 (m, 4H), 1.52-1.39 (m, 1H), 1.47-1.12 (m, 6H), 1.09 (s, 3H), 0.95-0.92 (m, H), 0.74 (s, 3H).
References:
WO2015/10054,2015,A2 Location in patent:Page/Page column 75; 76