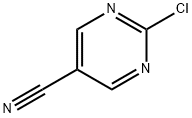
2-Chloropyrimidine-5-carbonitrile synthesis
- Product Name:2-Chloropyrimidine-5-carbonitrile
- CAS Number:1753-50-0
- Molecular formula:C5H2ClN3
- Molecular Weight:139.54
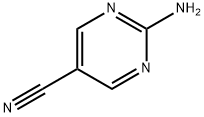
1753-48-6
141 suppliers
$31.00/1g

1753-50-0
165 suppliers
$18.00/250mg
Yield:1753-50-0 72%
Reaction Conditions:
Stage #1:2-aminopyrimidine-5-carbonitrile with tert.-butylnitrite in acetonitrile at 20; for 1 h;Inert atmosphere;
Stage #2: with copper dichloride in acetonitrile at 60; for 24 h;
Steps:
3 (3) synthesis of 2-chloropyrimidine-5-carbonitrile
A 500 mL two-necked flask was added with 5-bromopyrimidin-2-amine (5.18 g, 30 mmol), Zn(CN)2 (2.11 g, 18 mmol), Pd(PPh3)4 (3.46 g, 3 mmol), Zinc (0.045 g, 1.5 mmol), and Zn(OAc)2 (0.33 g, 1.5 mmol), equipped with a magnetic stirring bar, an addition funnel, and a condensing tube, and charged with argon after evacuation. Dry DMF (300 mL) was injected with a syringe, heated at 120° C. for 24 hours, allowed to cool down to RT, and poured into 1000 mL water. The white powder was precipitated, filtered, and washed with methanol, giving a white powder formed 2-aminopyrimidine-5-carbonitrile. Then, a 100 mL two-necked flask was added with 2-aminopyrimidine-5-carbonitrile (3.6 g, 30 mmol), tert-butyl nitrite (BuNO) (5.35 mL, 43 mmol), equipped with a magnetic stirring bar, an addition funnel, and a condensing tube, and charged with argon after evacuation. Acetonitrile (60 mL) was firstly injected with a syringe, stirred at RT for 1 hour, and then a solid CuCl2 was added, heated at 60° C. for 24 hours. After cool down to RT, 20 mL diethyl ether was added and filtered. After the filtrate was subjected to rotary concentration to remove excess solvent, the solid was purified by column chromatography (SiO2, Dichloromethane/Hexane=1/1), giving a white solid (3 g, 22 mmol, 72%). 1H NMR (400 MHz, CDCl3) δ ppm 8.90 (s, 2H). The preparation process is shown below.
References:
AU OPTRONICS CORPORATION;HU, Hung-Chieh;CHEN, Chung-Chia;LEE, Meng-Ting;HO, Yu-Yi;LI, Shu-Wei;WONG, Ken-Tsung;PAN, Kuan-Chung;SHIU, Yi-Jiun;TSAI, Wei-Lung;WU, Chung-Chih US2017/170419, 2017, A1 Location in patent:Paragraph 0041
856595-94-3
23 suppliers
inquiry

1753-50-0
165 suppliers
$18.00/250mg
38373-47-6
42 suppliers
inquiry

1753-50-0
165 suppliers
$18.00/250mg
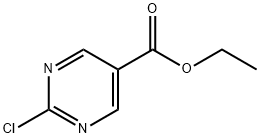
89793-12-4
241 suppliers
$6.00/100mg

1753-50-0
165 suppliers
$18.00/250mg
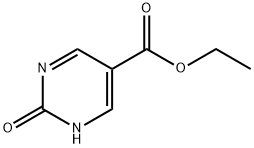
95928-49-7
96 suppliers
$45.00/50mg

1753-50-0
165 suppliers
$18.00/250mg